Ligand Controlled Ir-Catalyzed Regiodivergent Oxyamination of Unactivated Alkenes
- PMID: 31310537
- PMCID: PMC6980349
- DOI: 10.1021/jacs.9b06366
Ligand Controlled Ir-Catalyzed Regiodivergent Oxyamination of Unactivated Alkenes
Abstract
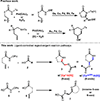
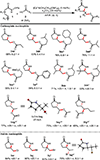
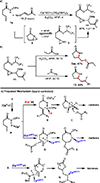
An intramolecular Ir(III)-catalyzed regiodivergent oxyamination of unactivated alkenes provides valuable γ-lactams, γ-lactones and δ-lactams. The regioselectivity is controlled by the electronically tunable cyclopentadienyl Ir(III)-complexes enabling oxyamination via either 5-exo or 6-endo pathways. With respect to the mechanism, we propose a highly reactive [3.1.0] bicycle intermediate derived from Ir(V) nitrene-mediated aziridination to be a key intermediate toward the synthesis of γ-lactams.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Donohoe TJ; Callens CKA; Flores A; Lacy AR; Rathi AH, Recent Developments in Methodology for the Direct Oxyamination of Olefins. Chem. - Eur. J 2011, 17, 58. - PubMed
-
- Sharpless KB; Chong AO; Oshima K, Osmium-catalyzed vicinal oxyamination of olefins by Chloramine-T. J. Org. Chem 1976, 41, 177;
- Herranz E; Sharpless KB, Improvements in the osmium-catalyzed oxyamination of olefins by chloramine-T J. Org. Chem 1978, 43, 2544;
- Li G; Chang H-T; Sharpless KB, Catalytic Asymmetric Aminohydroxylation (AA) of Olefins. Angew. Chem., Int. Ed 1996, 35, 451;
- O’Brien P, Sharpless Asymmetric Aminohydroxylation: Scope, Limitations, and Use in Synthesis. Angew. Chem. Int. Ed 1999, 38, 326 and references therein. - PubMed
-
- Morgan AJ; Masse CE; Panek JS, Reversal of Regioselection in the Sharpless Asymmetric Aminohydroxylation of Aryl Ester Substrates. Org. Lett 1999, 1, 1949; - PubMed
- Donohoe TJ; Johnson PD; Cowley A; Keenan M, The Tethered Aminohydroxylation (TA) of Cyclic Allylic Carbamates. J. Am. Chem. Soc 2002, 124, 12934; - PubMed
- Donohoe TJ; Chughtai MJ; Klauber DJ; Griffin D; Campbell AD, N-Sulfonyloxy Carbamates as Reoxidants for the Tethered Aminohydroxylation Reaction. J. Am. Chem. Soc 2006, 128, 2514; - PubMed
- Donohoe TJ; Bataille CJR; Gattrell W; Kloesges J; Rossignol E, Tethered Aminohydroxylation: Dramatic Improvements to the Process. Org. Lett 2007, 9, 1725; - PubMed
- Donohoe TJ; Callens CKA; Thompson AL Tethered Aminohydroxylation (TA) Reaction of Amides. Org. Lett 2009, 11, 2306; - PubMed
- Donohoe TJ; Callens CKA; Flores A; Mesch S; Poole DL; Roslan IA, Amino Acid-Based Reoxidants for Aminohydroxylation: Application to the Construction of Amino Acid-Amino Alcohol Conjugates. Angew. Chem., Int. Ed 2011, 50, 10957; - PubMed
- Ma Z; Naylor BC; Loertscher BM; Hafen DD; Li JM; Castle SL, Regioselective Base-Free Intermolecular Aminohydroxylations of Hindered and Functionalized Alkenes. J. Org. Chem 2012, 77, 1208. - PubMed
-
- Noack M; Gottlich R, Copper(I) catalysed cyclisation of unsaturated N-benzoyloxyamines: an aminohydroxylation via radicals. Chem. Commun 2002, 536; - PubMed
- Michaelis DJ; Shaffer CJ; Yoon TP, Copper(II)-Catalyzed Aminohydroxylation of Olefins. J. Am. Chem. Soc 2007, 129, 1866; - PubMed
- Fuller PH; Kim J-W; Chemler SR, Copper Catalyzed Enantioselective Intramolecular Aminooxygenation of Alkenes. J. Am. Chem. Soc 2008, 130, 17638; - PMC - PubMed
- Mancheno DE; Thornton AR; Stoll AH; Kong A; Blakey SB, Copper-Catalyzed Olefin Aminoacetoxylation. Org. Lett 2010, 12, 4110; - PubMed
- Nakanishi M; Minard C; Retailleau P; Cariou K; Dodd RH, Copper(I) catalyzed regioselective asymmetric alkoxyamination of aryl enamide derivatives. Org. Lett 2011, 13, 5792; - PubMed
- Sequeira FC; Chemler SR, Stereoselective Synthesis of Morpholines via Copper-Promoted Oxyamination of Alkenes. Org. Lett 2012, 14, 4482; - PMC - PubMed
- Sanjaya S; Chiba S, Copper-catalyzed aminooxygenation of N-allylamidines with PhI(OAc)2. Org Lett 2012, 14, 5342; - PubMed
- Zhu R; Buchwald SL, Versatile Enantioselective Synthesis of Functionalized Lactones via Copper-Catalyzed Radical Oxyfunctionalization of Alkenes. J. Am. Chem. Soc 2015, 137, 8069; - PMC - PubMed
- Hemric BN; Shen K; Wang Q, Copper-Catalyzed Amino Lactonization and Amino Oxygenation of Alkenes Using O-Benzoylhydroxylamines. J. Am. Chem. Soc 2016, 138, 5813; - PubMed
- Liu R-H; Wei D; Han B; Yu W, Copper-Catalyzed Oxidative Oxyamination/Diamination of Internal Alkenes of Unsaturated Oximes with Simple Amines. ACS Catal. 2016, 6, 6525;
- Ren S; Song S; Ye L; Feng C; Loh T-P, Copper-catalyzed oxyamination of electron-deficient alkenes with N-acyloxyamines. Chem. Commun 2016, 52, 10373; - PubMed
- Chemler SR; Karyakarte SD; Khoder ZM, Stereoselective and Regioselective Synthesis of Heterocycles via Copper-Catalyzed Additions of Amine Derivatives and Alcohols to Alkenes. J. Org. Chem 2017, 82, 11311; - PMC - PubMed
- Xie J; Wang YW; Qi LW; Zhang B, Access to Aminated Saturated Oxygen Heterocycles via Copper-Catalyzed Aminooxygenation of Alkenes. Org Lett 2017, 19, 1148; - PubMed
- Wu F; Stewart S; Ariyarathna JP; Li W, Aerobic Copper-Catalyzed Alkene Oxyamination for Amino Lactone Synthesis. ACS Catal. 2018, 8, 1921.
-
- Bäckvall JE; Björkman EE, Stereospecific Palladium-Promoted Oxyamination of Alkenes. J. Org. Chem 1980, 45, 2893;
- Alexanian EJ; Lee C; Sorensen EJ, Palladium-Catalyzed Ring-Forming Aminoacetoxylation of Alkenes. J. Am. Chem. Soc 2005, 127, 7690; - PubMed
- Liu G; Stahl SS, Highly Regioselective Pd-Catalyzed Intermolecular Aminoacetoxylation of Alkenes and Evidence for cis-Aminopalladation and SN2 C-O Bond Formation. J. Am. Chem. Soc 2006, 128, 7179; - PubMed
- Desai LV; Sanford MS, Construction of Tetrahydrofurans by PdII/PdIV-Catalyzed Aminooxygenation of Alkenes. Angew. Chem., Int. Ed 2007, 46, 5737; - PubMed
- Zhu H; Chen P; Liu G, Pd-Catalyzed Intramolecular Aminohydroxylation of Alkenes with Hydrogen Peroxide as Oxidant and Water as Nucleophile. J. Am. Chem. Soc 2014, 136, 1766; - PubMed
- Rao WH; Yin XS; Shi BF, Catalyst-Controlled Amino-versus Oxy-Acetoxylation of Urea-Tethered Alkenes: Efficient Synthesis of Cyclic Ureas and Isoureas. Org. Lett 2015, 17, 3758. - PubMed
- Qi X; Chen C; Hou C; Fu L; Chen P; Liu G, Enantioselective Pd(II)-Catalyzed Intramolecular Oxidative 6-endo Aminoacetoxylation of Unactivated Alkenes. J. Am. Chem. Soc 2018, 140, 7415. - PubMed
- Zeng T; Liu Z; Schmidt MA; Eastgate MD; Engle KM, Directed, Palladium(II)-Catalyzed Intermolecular Aminohydroxylation of Alkenes Using a Mild Oxidation System. Org Lett 2018, 20, 3853; - PMC - PubMed
- Shen HC; Wu YF; Zhang Y; Fan LF; Han ZY; Gong LZ, Palladium-Catalyzed Asymmetric Aminohydroxylation of 1,3-Dienes. Angew. Chem. Int. Ed 2018, 57, 2372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

