The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis
- PMID: 31310589
- PMCID: PMC6763233
- DOI: 10.1172/JCI128654
The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis
Abstract
Background: Checkpoint inhibitor pneumonitis (CIP) is a highly morbid complication of immune checkpoint immunotherapy (ICI), one which precludes the continuation of ICI. Yet, the mechanistic underpinnings of CIP are unknown.
Methods: To better understand the mechanism of lung injury in CIP, we prospectively collected bronchoalveolar lavage (BAL) samples in ICI-treated patients with (n=12) and without CIP (n=6), prior to initiation of first-line therapy for CIP (high dose corticosteroids. We analyzed BAL immune cell populations using a combination of traditional multicolor flow cytometry gating, unsupervised clustering analysis and BAL supernatant cytokine measurements.
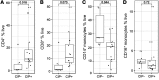
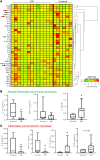
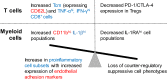
Results: We found increased BAL lymphocytosis, predominantly CD4+ T cells, in CIP. Specifically, we observed increased numbers of BAL central memory T-cells (Tcm), evidence of Type I polarization, and decreased expression of CTLA-4 and PD-1 in BAL Tregs, suggesting both activation of pro-inflammatory subsets and an attenuated suppressive phenotype. CIP BAL myeloid immune populations displayed enhanced expression of IL-1β and decreased expression of counter-regulatory IL-1RA. We observed increased levels of T cell chemoattractants in the BAL supernatant, consistent with our pro-inflammatory, lymphocytic cellular landscape.
Conclusion: We observe several immune cell subpopulations that are dysregulated in CIP, which may represent possible targets that could lead to therapeutics for this morbid immune related adverse event.
Keywords: Cancer immunotherapy; Immunology; Lung cancer; Pulmonology; Th1 response.
Conflict of interest statement
Figures






Comment in
-
Recent Insights into In Utero Gene Editing, Checkpoint Inhibitors, and Polymorphonuclear Extracellular Vesicles.Am J Respir Cell Mol Biol. 2020 Jun;62(6):805-807. doi: 10.1165/rcmb.2020-0024RO. Am J Respir Cell Mol Biol. 2020. PMID: 32017596 No abstract available.
References
-
- Balaji A, Verde F, Suresh K, Naidoo J. Pneumonitis from anti-PD-1/ PD-L1 therapy. Oncology (Williston Park, NY) 2017;31(10):739–746, 754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

