Intranasal sufentanil versus intravenous morphine for acute severe trauma pain: A double-blind randomized non-inferiority study
- PMID: 31310600
- PMCID: PMC6634380
- DOI: 10.1371/journal.pmed.1002849
Intranasal sufentanil versus intravenous morphine for acute severe trauma pain: A double-blind randomized non-inferiority study
Abstract
Background: Intravenous morphine (IVM) is the most common strong analgesic used in trauma, but is associated with a clear time limitation related to the need to obtain an access route. The intranasal (IN) route provides easy administration with a fast peak action time due to high vascularization and the absence of first-pass metabolism. We aimed to determine whether IN sufentanil (INS) for patients presenting to an emergency department with acute severe traumatic pain results in a reduction in pain intensity non-inferior to IVM.
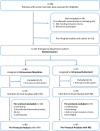
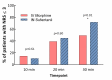
Methods and findings: In a prospective, randomized, multicenter non-inferiority trial conducted in the emergency departments of 6 hospitals across France, patients were randomized 1:1 to INS titration (0.3 μg/kg and additional doses of 0.15 μg/kg at 10 minutes and 20 minutes if numerical pain rating scale [NRS] > 3) and intravenous placebo, or to IVM (0.1 mg/kg and additional doses of 0.05 mg/kg at 10 minutes and 20 minutes if NRS > 3) and IN placebo. Patients, clinical staff, and research staff were blinded to the treatment allocation. The primary endpoint was the total decrease on NRS at 30 minutes after first administration. The prespecified non-inferiority margin was -1.3 on the NRS. The primary outcome was analyzed per protocol. Adverse events were prospectively recorded during 4 hours. Among the 194 patients enrolled in the emergency department cohort between November 4, 2013, and April 10, 2016, 157 were randomized, and the protocol was correctly administered in 136 (69 IVM group, 67 INS group, per protocol population, 76% men, median age 40 [IQR 29 to 54] years). The mean difference between NRS at first administration and NRS at 30 minutes was -4.1 (97.5% CI -4.6 to -3.6) in the IVM group and -5.2 (97.5% CI -5.7 to -4.6) in the INS group. Non-inferiority was demonstrated (p < 0.001 with 1-sided mean-equivalence t test), as the lower 97.5% confidence interval of 0.29 (97.5% CI 0.29 to 1.93) was above the prespecified margin of -1.3. INS was superior to IVM (intention to treat analysis: p = 0.034), but without a clinically significant difference in mean NRS between groups. Six severe adverse events were observed in the INS group and 2 in the IVM group (number needed to harm: 17), including an apparent imbalance for hypoxemia (3 in the INS group versus 1 in the IVM group) and for bradypnea (2 in the INS group versus 0 in the IVM group). The main limitation of the study was that the choice of concomitant analgesics, when they were used, was left to the discretion of the physician in charge, and co-analgesia was more often used in the IVM group. Moreover, the size of the study did not allow us to conclude with certainty about the safety of INS in emergency settings.
Conclusions: We confirm the non-inferiority of INS compared to IVM for pain reduction at 30 minutes after administration in patients with severe traumatic pain presenting to an emergency department. The IN route, with no need to obtain a venous route, may allow early and effective analgesia in emergency settings and in difficult situations. Confirmation of the safety profile of INS will require further larger studies.
Trial registration: ClinicalTrials.gov NCT02095366. EudraCT 2013-001665-16.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: MM has received grants and personal fees from MundiPharma and Purdue for speaking at conferences, and support from Roche Diagnostics for research unrelated to that presented here; GD has received personal fees from Zoll Medical for speaking at conferences; DV has received grants from Mundipharma for speaking at conferences, and grants from Astra Zeneca for research unrelated to that presented here. All other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

