Design of bile-based vesicles (BBVs) for hepatocytes specific delivery of Daclatasvir: Comparison of ex-vivo transenterocytic transport, in-vitro protein adsorption resistance and HepG2 cellular uptake of charged and β-sitosterol decorated vesicles
- PMID: 31310613
- PMCID: PMC6634393
- DOI: 10.1371/journal.pone.0219752
Design of bile-based vesicles (BBVs) for hepatocytes specific delivery of Daclatasvir: Comparison of ex-vivo transenterocytic transport, in-vitro protein adsorption resistance and HepG2 cellular uptake of charged and β-sitosterol decorated vesicles
Abstract
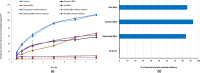
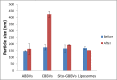
Daclatasvir is a new direct acting antiviral used in treatment of Hepatitis C virus, in an attempt to increase its hepatocytes specificity and uptake. It was encapsulated within bile based vesicles (BBVs) containing egg phosphatidyl choline, cholesterol and sodium deoxycholate fabricated by thin-film hydration method. A D-optimal mixture design was applied to study the effect of formulation variables on vesicular characteristics. The dependent variables picked were the particle size, polydispersity index, zeta potential and entrapment efficiency. The optimized bile based vesicles were subjected for further modifications to prepare miniaturized anionic (ABBVs), cationic (CBBVs) and Sito-G decorated BBVs (Sito-GBBVs) to be capable to penetrate liver fenestrae (<200 nm). The aim of the current work is to compare the potential of the ABBVs, CBBVs and Sito-GBBVs loaded with Daclatasvir for stability in simulated biological fluids, ex-vivo intestinal transenterocytic transport, HepG2 cellular uptake and resistance to blood protein adsorption. The miniaturized ABBVs, CBBVs and Sito-GBBVs showed acceptable stability in simulated biological fluids. CBBVs had the highest transenterocytic transport through intestinal membrane. The internalization of CBBVs into HepG2 cells was about 2.1 folds that of ABBVs and 1.45 folds that of Sito-GBBVs. ABBVs and Sito-GBBVs showed superior resistance to opsonization compared to CBBVs which showed significant increase in particle size (p˃0.05) due to protein adsorption. The miniaturized Sito-GBBVs constitute a promising strategy to overcome key biological barriers facing hepatocytes specific delivery of Daclatasvir.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

