Is Scientific Medical Literature Related to Endometriosis Treatment Evidence-Based? A Systematic Review on Methodological Quality of Randomized Clinical Trials
- PMID: 31311075
- PMCID: PMC6681304
- DOI: 10.3390/medicina55070372
Is Scientific Medical Literature Related to Endometriosis Treatment Evidence-Based? A Systematic Review on Methodological Quality of Randomized Clinical Trials
Abstract
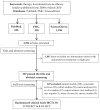
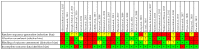
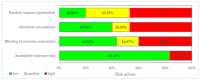
Background and objectives: Informed decision-making requires the ability to identify and integrate high-quality scientific evidence in daily practice. We aimed to assess whether randomized controlled trials (RCTs) on endometriosis therapy follow methodological criteria corresponding to the RCTs' specific level in the hierarchy of evidence in such details to allow the reproduction and replication of the study. Materials and Methods: Using the keywords "therapy" and "endometriosis" and "efficacy" three bibliographic databases were searched for English written scientific articles published from 1 January 2008 to 3 March 2018. Only the randomized clinical trials (RCTs) were evaluated in terms of whether they provided the appropriate level of scientific evidence, equivalent to level 1, degree 1b in the hierarchy of evidence. A list of criteria to ensure study replication and reproduction, considering CONSORT guideline and MECIR standards, was developed and used to evaluate RCTs' methodological soundness, and scores were granted. Three types of bias, namely selection bias (random sequence generation and allocation concealment), detection bias (blinding of outcome assessment), and attrition bias (incomplete outcome data) were also evaluated. Results: We found 387 articles on endometriosis therapy, of which 38 were RCTs: 30 double-blinded RCTs and 8 open-label RCTs. No article achieved the maximum score according to the evaluated methodological criteria. Even though 73.3% of the double-blinded RCTs had clear title, abstract, introduction, and objectives, only 13.3% provided precise information regarding experimental design and randomization, and also showed a low risk of bias. The blinding method was poorly reported in 43.3% of the double-blinded RCTs, while allocation concealment and random sequence generation were inadequate in 33.3% of them. Conclusions: None of the evaluated RCTs met all the methodological criteria, none had only a low risk of bias and provided sufficient details on methods and randomization to allow for the reproduction and replication of the study. Consequently, the appropriate level of scientific evidence (level 1, degree 1b) could not be granted. On endometriosis therapy, this study evaluated the quality of reporting in RCTs and not the quality of how the studies were performed.
Keywords: endometriosis therapy; hierarchy of evidence; randomized clinical trial; reporting quality; study replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Dodd S., Susanna D., Ian W., Paula W. Departure from treatment protocol in published randomised controlled trials: A review. Trials. 2011;12:A129. doi: 10.1186/1745-6215-12-S1-A129. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

