Human Platelet Lysate as a Functional Substitute for Fetal Bovine Serum in the Culture of Human Adipose Derived Stromal/Stem Cells
- PMID: 31311198
- PMCID: PMC6679214
- DOI: 10.3390/cells8070724
Human Platelet Lysate as a Functional Substitute for Fetal Bovine Serum in the Culture of Human Adipose Derived Stromal/Stem Cells
Abstract
Introduction: Adipose derived stromal/stem cells (ASCs) hold potential as cell therapeutics for a wide range of disease states; however, many expansion protocols rely on the use of fetal bovine serum (FBS) as a cell culture nutrient supplement. The current study explores the substitution of lysates from expired human platelets (HPLs) as an FBS substitute.
Methods: Expired human platelets from an authorized blood center were lysed by freeze/thawing and used to examine human ASCs with respect to proliferation using hematocytometer cell counts, colony forming unit-fibroblast (CFU-F) frequency, surface immunophenotype by flow cytometry, and tri-lineage (adipocyte, chondrocyte, osteoblast) differentiation potential by histochemical staining.
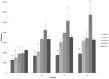
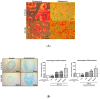
Results: The proliferation assays demonstrated that HPLs supported ASC proliferation in a concentration dependent manner, reaching levels that exceeded that observed in the presence of 10% FBS. The concentration of 0.75% HPLs was equivalent to 10% FBS when utilized in cell culture media with respect to proliferation, immunophenotype, and CFU-F frequency. When added to osteogenic, adipogenic, and chondrogenic differentiation media, both supplements showed appropriate differentiation by staining.
Conclusion: HPLs is an effective substitute for FBS in the culture, expansion and differentiation of human ASCs suitable for pre-clinical studies; however, additional assays and analyses will be necessary to validate HPLs for clinical applications and regulatory approval.
Keywords: adipogenesis; adipose-derived stromal/stem cells; chondrogenesis; colony forming unit-fibroblast; fetal bovine serum; human platelet lysate; mesenchymal stem cell; osteogenesis; regenerative medicine.
Conflict of interest statement
M.H.M. is an employee of Obatala Sciences. T.F. is a co-owner, co-founder, CEO and President of Obatala Sciences and former employee of LaCell. X.W. is co-owner and co-founder of LaCell and Obatala and serves as Vice President for R & D at LaCell. J.M.G. is co-owner and co-founder of LaCell and Obatala Sciences, serves as Chief Scientific Officer of LaCell. T.F., X.W., and J.M.G. are inventors on patents relevant to this field. The remaining authors have no disclosures to declare.
Figures


References
-
- Bourin P., Bunnell B.A., Casteilla L., Dominici M., Katz A.J., March K.L., Redl H., Rubin J.P., Yoshimura K., Gimble J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. - PMC - PubMed
-
- Kilroy G.E., Foster S.J., Wu X., Ruiz J., Sherwood S., Heifetz A., Ludlow J.W., Stricker D.M., Potiny S., Green P., et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J. Cell. Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

