Selection and characterization of FcεRI phospho-ITAM specific antibodies
- PMID: 31311408
- PMCID: PMC6748597
- DOI: 10.1080/19420862.2019.1632113
Selection and characterization of FcεRI phospho-ITAM specific antibodies
Abstract
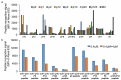
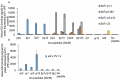
Post-translational modifications, such as the phosphorylation of tyrosines, are often the initiation step for intracellular signaling cascades. Pan-reactive antibodies against modified amino acids (e.g., anti-phosphotyrosine), which are often used to assay these changes, require isolation of the specific protein prior to analysis and do not identify the specific residue that has been modified (in the case that multiple amino acids have been modified). Phosphorylation state-specific antibodies (PSSAs) developed to recognize post-translational modifications within a specific amino acid sequence can be used to study the timeline of modifications during a signal cascade. We used the FcεRI receptor as a model system to develop and characterize high-affinity PSSAs using phage and yeast display technologies. We selected three β-subunit antibodies that recognized: 1) phosphorylation of tyrosines Y218 or Y224; 2) phosphorylation of the Y228 tyrosine; and 3) phosphorylation of all three tyrosines. We used these antibodies to study the receptor activation timeline of FcεR1 in rat basophilic leukemia cells (RBL-2H3) upon stimulation with DNP24-BSA. We also selected an antibody recognizing the N-terminal phosphorylation site of the γ-subunit (Y65) of the receptor and applied this antibody to evaluate receptor activation. Recognition patterns of these antibodies show different timelines for phosphorylation of tyrosines in both β and γ subunits. Our methodology provides a strategy to select antibodies specific to post-translational modifications and provides new reagents to study mast cell activation by the high-affinity IgE receptor, FcεRI.
Keywords: FcεR1 receptor subunits; Phosphorylation state-specific antibodies; phage display; post-translational modifications; yeast display.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
