Systematic analysis of the lysine succinylome in the model medicinal mushroom Ganoderma lucidum
- PMID: 31311503
- PMCID: PMC6636155
- DOI: 10.1186/s12864-019-5962-0
Systematic analysis of the lysine succinylome in the model medicinal mushroom Ganoderma lucidum
Abstract
Background: Ganoderma lucidum, one of the best-known medicinal mushrooms in the world, produces more than 400 different bioactive compounds. However, the regulation of these bioactive compounds biosynthesis is still unclear. Lysine succinylation is a critical post-translational modification and has many important functions in all aspects of eukaryotic and prokaryotic cells. Although it has been studied for a long time, its function is still unclear in G. lucidum. In this study, a global investigation was carried out on the succinylome in G. lucidum.
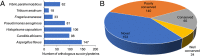
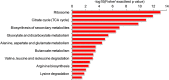
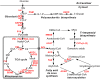
Results: In total, 382 modified proteins which contain 742 lysine succinylated sites were obtained. The proteomics data are available through ProteomeXchange with the dataset accession number PXD013954. Bioinformatics analysis revealed that the succinylated proteins were distributed in various cellular biological processes and participated in a great variety of metabolic pathways including carbon metabolism and biosynthesis of secondary metabolites. Notably, a total of 47 enzymes associated with biosynthesis of triterpenoids and polysaccharides were found to be succinylated. Furthermore, two succinylated sites K90 and K106 were found in the conserved Fve region of immunomodulatory protein LZ8. These observations show that lysine succinylation plays an indispensable role in metabolic regulation of bioactive compounds in G. lucidum.
Conclusions: These findings indicate that lysine succinylation is related to many metabolic pathways, especially pharmacologically bioactive compounds metabolism. This study provides the first global investigation of lysine succinylation in G. lucidum and the succinylome dataset provided in this study serves as a resource to further explore the physiological roles of these modifications in secondary metabolism.
Keywords: Ganoderma lucidum; Immunomodulatory protein; Polysaccharides; Post-translational modification; Succinylome; Triterpenoids.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
The first succinylome profile of Trichophyton rubrum reveals lysine succinylation on proteins involved in various key cellular processes.BMC Genomics. 2017 Aug 4;18(1):577. doi: 10.1186/s12864-017-3977-y. BMC Genomics. 2017. PMID: 28778155 Free PMC article.
-
First succinyl-proteome profiling of extensively drug-resistant Mycobacterium tuberculosis revealed involvement of succinylation in cellular physiology.J Proteome Res. 2015 Jan 2;14(1):107-19. doi: 10.1021/pr500859a. Epub 2014 Nov 12. J Proteome Res. 2015. PMID: 25363132
-
Systematic Analysis of the Lysine Succinylome in Candida albicans.J Proteome Res. 2016 Oct 7;15(10):3793-3801. doi: 10.1021/acs.jproteome.6b00578. Epub 2016 Sep 21. J Proteome Res. 2016. PMID: 27605073
-
Therapeutic potential and nutritional significance of Ganoderma lucidum - a comprehensive review from 2010 to 2022.Food Funct. 2023 Feb 21;14(4):1812-1838. doi: 10.1039/d2fo01683d. Food Funct. 2023. PMID: 36734035
-
Cellular and physiological effects of Ganoderma lucidum (Reishi).Mini Rev Med Chem. 2004 Oct;4(8):873-9. doi: 10.2174/1389557043403323. Mini Rev Med Chem. 2004. PMID: 15544548 Review.
Cited by
-
Systematic analysis of the lysine malonylome in Sanghuangporus sanghuang.BMC Genomics. 2021 Nov 19;22(1):840. doi: 10.1186/s12864-021-08120-0. BMC Genomics. 2021. PMID: 34798813 Free PMC article.
-
Global Identification and Systematic Analysis of Lysine Malonylation in Maize (Zea mays L.).Front Plant Sci. 2021 Aug 20;12:728338. doi: 10.3389/fpls.2021.728338. eCollection 2021. Front Plant Sci. 2021. PMID: 34490025 Free PMC article.
-
Impact of Lysine Succinylation on the Biology of Fungi.Curr Issues Mol Biol. 2024 Jan 23;46(2):1020-1046. doi: 10.3390/cimb46020065. Curr Issues Mol Biol. 2024. PMID: 38392183 Free PMC article. Review.
-
Post-translational modifications drive secondary metabolite biosynthesis in Aspergillus: a review.Environ Microbiol. 2022 Jul;24(7):2857-2881. doi: 10.1111/1462-2920.16034. Epub 2022 May 30. Environ Microbiol. 2022. PMID: 35645150 Free PMC article. Review.
-
Label-Free Comparative Proteomics Analysis Revealed Heat Stress Responsive Mechanism in Hypsizygus marmoreus.Front Microbiol. 2021 Jan 5;11:541967. doi: 10.3389/fmicb.2020.541967. eCollection 2020. Front Microbiol. 2021. PMID: 33469447 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

