Improving preparedness to respond to cross-border hepatitis A outbreaks in the European Union/European Economic Area: towards comparable sequencing of hepatitis A virus
- PMID: 31311618
- PMCID: PMC6636214
- DOI: 10.2807/1560-7917.ES.2019.24.28.1800397
Improving preparedness to respond to cross-border hepatitis A outbreaks in the European Union/European Economic Area: towards comparable sequencing of hepatitis A virus
Abstract
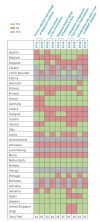
IntroductionSequence-based typing of hepatitis A virus (HAV) is important for outbreak detection, investigation and surveillance. In 2013, sequencing was central to resolving a large European Union (EU)-wide outbreak related to frozen berries. However, as the sequenced HAV genome regions were only partly comparable between countries, results were not always conclusive.AimThe objective was to gather information on HAV surveillance and sequencing in EU/European Economic Area (EEA) countries to find ways to harmonise their procedures, for improvement of cross-border outbreak responses.MethodsIn 2014, the European Centre for Disease Prevention and Control (ECDC) conducted a survey on HAV surveillance practices in EU/EEA countries. The survey enquired whether a referral system for confirming primary diagnostics of hepatitis A existed as well as a central collection/storage of hepatitis A cases' samples for typing. Questions on HAV sequencing procedures were also asked. Based on the results, an expert consultation proposed harmonised procedures for cross-border outbreak response, in particular regarding sequencing. In 2016, a follow-up survey assessed uptake of suggested methods.ResultsOf 31 EU/EEA countries, 23 (2014) and 27 (2016) participated. Numbers of countries with central collection and storage of HAV positive samples and of those performing sequencing increased from 12 to 15 and 12 to 14 respectively in 2016, with all countries typing an overlapping fragment of 218 nt. However, variation existed in the sequenced genomic regions and their lengths.ConclusionsWhile HAV sequences in EU/EEA countries are comparable for surveillance, collaboration in sharing and comparing these can be further strengthened.
Keywords: European Union; HAV; capacity building; foodborne diseases; hepatitis A virus; sequence analysis; surveys and questionnaires.
Conflict of interest statement
Figures



Comment in
-
Persisting higher prevalence of hepatitis A virus RNA in blood donors, France, 2018.Euro Surveill. 2019 Nov;24(47):1900695. doi: 10.2807/1560-7917.ES.2019.24.47.1900695. Euro Surveill. 2019. PMID: 31771700 Free PMC article. No abstract available.
References
-
- World Health Organization (WHO) WHO position paper on hepatitis A vaccines – June 2012. Wkly Epidemiol Rec. 2012;87(28/29):261-76. - PubMed
-
- Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545-602. 10.1016/S0140-6736(16)31678-6 - DOI - PMC - PubMed
-
- European Centre for Disease Prevention and Control (ECDC). Hepatitis A. In: ECDC. Annual epidemiological report for 2016. Stockholm: ECDC; 2019. Available from: https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2016-hepatit...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
