Cholesterol and 27-hydroxycholesterol promote thyroid carcinoma aggressiveness
- PMID: 31311983
- PMCID: PMC6635382
- DOI: 10.1038/s41598-019-46727-2
Cholesterol and 27-hydroxycholesterol promote thyroid carcinoma aggressiveness
Abstract
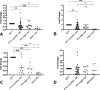
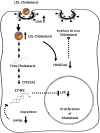
Cholesterol mediates its proliferative and metastatic effects via the metabolite 27-hydroxycholesterol (27-HC), at least in breast and endometrial cancer. We determined the serum lipoprotein profile, intratumoral cholesterol and 27-HC levels in a cohort of patients with well-differentiated papillary thyroid carcinoma (PTC; low/intermediate and high risk), advanced thyroid cancers (poorly differentiated, PDTC and anaplastic thyroid carcinoma, ATC) and benign thyroid tumors, as well as the expression of genes involved in cholesterol metabolism. We investigated the gene expression profile, cellular proliferation, and migration in Nthy-ori 3.1 and CAL-62 cell lines loaded with human low-density lipoprotein (LDL). Patients with more aggressive tumors (high-risk PTC and PDTC/ATC) showed a decrease in blood LDL cholesterol and apolipoprotein B. These changes were associated with an increase in the expression of the thyroid's LDL receptor, whereas 3-hydroxy-3-methylglutaryl-CoA reductase and 25-hydroxycholesterol 7-alpha-hydroxylase were downregulated, with an intratumoral increase of the 27-HC metabolite. Furthermore, LDL promoted proliferation in both the Nthy-ori 3.1 and CAL-62 thyroid cellular models, but only in ATC cells was its cellular migration increased significantly. We conclude that cholesterol and intratumoral accumulation of 27-HC promote the aggressive behavior process of PTC. Targeting cholesterol metabolism could be a new therapeutic strategy in thyroid tumors with poor prognosis.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

