The NF2 tumor suppressor merlin interacts with Ras and RasGAP, which may modulate Ras signaling
- PMID: 31312020
- PMCID: PMC6756068
- DOI: 10.1038/s41388-019-0883-6
The NF2 tumor suppressor merlin interacts with Ras and RasGAP, which may modulate Ras signaling
Abstract
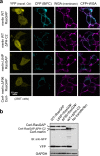
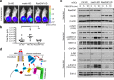
Inactivation of the tumor suppressor NF2/merlin underlies neurofibromatosis type 2 (NF2) and some sporadic tumors. Previous studies have established that merlin mediates contact inhibition of proliferation; however, the exact mechanisms remain obscure and multiple pathways have been implicated. We have previously reported that merlin inhibits Ras and Rac activity during contact inhibition, but how merlin regulates Ras activity has remained elusive. Here we demonstrate that merlin can directly interact with both Ras and p120RasGAP (also named RasGAP). While merlin does not increase the catalytic activity of RasGAP, the interactions with Ras and RasGAP may fine-tune Ras signaling. In vivo, loss of RasGAP in Schwann cells, unlike the loss of merlin, failed to promote tumorigenic growth in an orthotopic model. Therefore, modulation of Ras signaling through RasGAP likely contributes to, but is not sufficient to account for, merlin's tumor suppressor activity. Our study provides new insight into the mechanisms of merlin-dependent Ras regulation and may have additional implications for merlin-dependent regulation of other small GTPases.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

