GS-CA Compounds: First-In-Class HIV-1 Capsid Inhibitors Covering Multiple Grounds
- PMID: 31312185
- PMCID: PMC6613529
- DOI: 10.3389/fmicb.2019.01227
GS-CA Compounds: First-In-Class HIV-1 Capsid Inhibitors Covering Multiple Grounds
Abstract
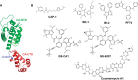
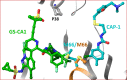
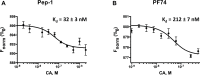
Recently reported HIV-1 capsid (CA) inhibitors GS-CA1 and GS-6207 (an analog of GS-CA1) are first-in-class compounds with long-acting potential. Reportedly, both compounds have greater potency than currently approved anti-HIV drugs. Due to the limited access to experimental data and the compounds themselves, a detailed mechanism of their inhibition is yet to be delineated. Using crystal structures of capsid-hexamers bound to well-studied capsid inhibitor PF74 and molecular modeling, we predict that GS-CA compounds bind in the pocket that is shared by previously reported CA inhibitors and host factors. Additionally, comparative modeling suggests that GS-CA compounds have unique structural features contributing to interactions with capsid. To test their proposed binding mode, we also report the design of a cyclic peptide combining structural units from GS-CA compounds, host factors, and previously reported capsid inhibitors. This peptide (Pep-1) binds CA-hexamer with a docking score comparable to GS-CA compounds. Affinity determination by MicroScale thermophoresis (MST) assays showed that CA binds Pep-1 with a ~7-fold better affinity than well-studied capsid inhibitor PF74, suggesting that it can be developed as a possible CA inhibitor.
Keywords: assembly; capsid; disassembly; human immunodeficiency virus; inhibitors; small molecules; uncoating.
Figures

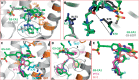
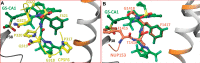
References
-
- ACD/ChemSketch (Freeware), version 2017.1.2. (2017). Toronto, ON, Canada: Advanced Chemistry Devlopment, Inc. Available at: www.acdlabs.com (Accessed June 22, 2017).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

