The application of graphene-based biomaterials in biomedicine
- PMID: 31312342
- PMCID: PMC6614642
The application of graphene-based biomaterials in biomedicine
Abstract
Graphene-based nanocomposites have attracted more and more attention recently in the field of biology and biomedicine. Graphene and its derivatives have been integrated with drugs, nucleic acids, antibodies, and other molecules. And these materials could be use as nanocomposite carriers or scaffold materials taking advantages of their enormous specific surface area, good elasticity and ductility, excellent biocompatibility, and outstanding mechanical strength. In addition, these composites have strong near-infrared absorbance and can act as photothermal agents to kill target cells through physical or chemical mechanisms. Along with significant advances in cell and organ transplantation, many of these materials have been explored in recent years for use in tissue engineering and regenerative medicine. Tissue engineering includes bone, nerve, heart, and muscle tissue engineering based on two-dimensional and three-dimensional graphene-based matrices or scaffolds possessing certain mechanical strengths and electrical conductivities, and the aim is to produce bioactive tissues to replace or repair natural tissue by promoting osteogenic, neuronal, and myogenic differentiation and myocardial cell growth. In this review, the basic properties of graphene-based complexes are systematically described and the biomedical applications of graphene-based materials in vivo and in vitro are summarized. This review first discusses the safety of graphene-based materials in terms of their biocompatibility and toxicity, and then it discusses these materials' applications in biosensing, photothermal therapy, stem cell culture, and tissue engineering. This review therefore provides a comprehensive understanding of graphene and its derivatives and their present and future applications.
Keywords: Graphene; biocompatibility; biomedical; tissue engineering; toxicity.
Conflict of interest statement
None.
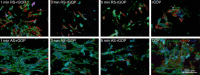
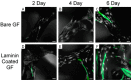
Figures



References
-
- Geim AK. Graphene: status and prospects. Science. 2009;324:1530–1534. - PubMed
-
- Nair RR, Blake P, Grigorenko AN, Novoselov KS, Booth TJ, Stauber T, Peres NM, Geim AK. Fine structure constant defines visual transparency of graphene. Science. 2008;320:104–106. - PubMed
-
- Tromp RM, Hannon JB. Thermodynamics and kinetics of graphene growth on SiC. PRL. 2009;102:106104. - PubMed
-
- Sutter P. Epitaxial graphene: how silicon leaves the scene. Nat Mater. 2009;8:171. - PubMed
-
- Kim KS, Zhao Y, Jang H, Lee SY, Kim JM, Kim KS, Ahn JH, Kim P, Choi JY, Hong BH. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature. 2009;457:706–710. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
