MiR-9 functions as a tumor suppressor in acute myeloid leukemia by targeting CX chemokine receptor 4
- PMID: 31312352
- PMCID: PMC6614627
MiR-9 functions as a tumor suppressor in acute myeloid leukemia by targeting CX chemokine receptor 4
Abstract
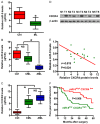
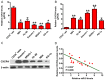
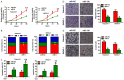
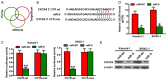
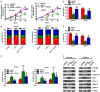
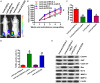
MicroRNAs (miRNAs) play key roles in the pathogenesis of many cancers, including acute myeloid leukemia (AML). Although miRNA-9 (miR-9) is involved in the leukemogenesis of AML, the underlying mechanisms remain to be elucidated. In this study, we found that miR-9 and C-X-C chemokine receptor 4 (CXCR4) were differentially expressed in myeloid leukemia, particularly in AML. The inverse correlation between miR-9 and CXCR4 was identified in AML samples and cell lines. The AML patients simultaneously with high levels of CXCR4 and low expression of miR-9 possessed poor prognosis. In vitro, miR-9 inhibited the proliferation, apoptosis resistance, migration, and invasion of AML cells. Dual luciferase assays verified CXCR4 as a direct target of miR-9. The suppressive effects of miR-9 on AML cells were counteracted or mimicked by CXCR4 overexpression or depletion, respectively. Overall, this study reveals that miR-9 retards the aggressive behaviors of AML cells by repressing CXCR4. Thus, miR-9/CXCR4 axis may represent a potential therapeutic target for AML.
Keywords: C-X-C chemokine receptor 4; acute myeloid leukemia; apoptosis; growth; microRNA-9.
Conflict of interest statement
None.
Figures
References
-
- Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. - PubMed
-
- Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. - PubMed
-
- Buyse M, Michiels S, Squifflet P, Lucchesi KJ, Hellstrand K, Brune ML, Castaigne S, Rowe JM. Leukemia-free survival as a surrogate end point for overall survival in the evaluation of maintenance therapy for patients with acute myeloid leukemia in complete remission. Haematologica. 2011;96:1106–1112. - PMC - PubMed
-
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. - PubMed
LinkOut - more resources
Full Text Sources
