Advances in the Physicochemical Profiling of Opioid Compounds of Therapeutic Interest
- PMID: 31312587
- PMCID: PMC6610444
- DOI: 10.1002/open.201900115
Advances in the Physicochemical Profiling of Opioid Compounds of Therapeutic Interest
Abstract
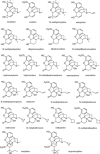
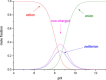
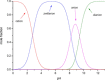
This review focuses on recent developments in the physicochemical profiling of morphine and other opioids. The acid-base properties and lipophilicity of these compounds is discussed at the microscopic, species-specific level. Examples are provided where this type of information can reveal the mechanism of pharmacokinetic processes at the submolecular level. The role of lipophilicity in quantitative structure-activity relationship (QSAR) studies of opioids is reviewed. The physicochemical properties and pharmacology of the main metabolites of morphine are also discussed. Recent studies indicate that the active metabolite morphine-6-glucuronide (M6G) can contribute to the analgesic activity of systemically administered morphine. The unexpectedly high lipophilicity of M6G partly accounts for its analgesic activity. When administered parenterally, another suspected minor metabolite, morphine-6-sulfate (M6S) has superior antinociceptive effects to those of morphine. However, because sulfate esters of morphine derivatives cannot cross the blood-brain barrier these esters may be good candidates to develop peripheral analgesic drugs.
Keywords: basicity; glucuronide conjugates; lipophilicity; opioids; physicochemical profiling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


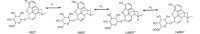

References
-
- Liu X., Smith B. J., Chen C., Callegari E., Becker S. L., Chen X., Cianfrogna J., Doran A. C., Doran S. D., Gibbs J. P., Hosea N., Liu J., Nelson F., Szewc M. A., Deusen J. V., J. Pharmacol. Exp. Ther. 2005, 313, 1254–1262. - PubMed
-
- Rankovic Z., J. Med. Chem. 2015, 58, 2584–2608. - PubMed
-
- Avdeef A., Barrett D. A., Shaw P. N., . Knaggs R. D., Davis S. S., J. Med. Chem. 1996, 39, 4377–4381. - PubMed
-
- Mazák K., Hosztafi S., Rácz Á., Noszál B., Mini-Rev. Med. Chem. 2009, 9, 984–995. - PubMed
-
- Mazák K., Noszál B., Eur. J. Pharm. Sci. 2014, 62, 96–104. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

