Rapamycin Rescues Age-Related Changes in Muscle-Derived Stem/Progenitor Cells from Progeroid Mice
- PMID: 31312666
- PMCID: PMC6610712
- DOI: 10.1016/j.omtm.2019.05.011
Rapamycin Rescues Age-Related Changes in Muscle-Derived Stem/Progenitor Cells from Progeroid Mice
Abstract
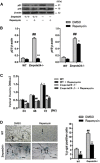
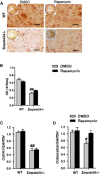
Aging-related loss of adult stem cell function contributes to impaired tissue regeneration. Mice deficient in zinc metalloproteinase STE24 (Zmpste24 -/-) exhibit premature age-related musculoskeletal pathologies similar to those observed in children with Hutchinson-Gilford progeria syndrome (HGPS). We have reported that muscle-derived stem/progenitor cells (MDSPCs) isolated from Zmpste24 -/- mice are defective in their proliferation and differentiation capabilities in culture and during tissue regeneration. The mechanistic target of rapamycin complex 1 (mTORC1) regulates cell growth, and inhibition of the mTORC1 pathway extends the lifespan of several animal species. We therefore hypothesized that inhibition of mTORC1 signaling would rescue the differentiation defects observed in progeroid MDSPCs. MDSPCs were isolated from Zmpste24 -/- mice, and the effects of mTORC1 on MDSPC differentiation and function were examined. We found that mTORC1 signaling was increased in senescent Zmpste24 -/- MDSPCs, along with impaired chondrogenic, osteogenic, and myogenic differentiation capacity versus wild-type MDSPCs. Interestingly, we observed that mTORC1 inhibition with rapamycin improved myogenic and chondrogenic differentiation and reduced levels of apoptosis and senescence in Zmpste24 -/- MDSPCs. Our results demonstrate that age-related adult stem/progenitor cell dysfunction contributes to impaired regenerative capacities and that mTORC1 inhibition may represent a potential therapeutic strategy for improving differentiation capacities of senescent stem and muscle progenitor cells.
Keywords: MDSPC; mTORC1; multipotent differentiation; muscle regeneration; rapamycin; vaging.
Figures






References
-
- Kirkwood T.B. Understanding the odd science of aging. Cell. 2005;120:437–447. - PubMed
-
- Vijg J. The role of DNA damage and repair in aging: new approaches to an old problem. Mech. Ageing Dev. 2008;129:498–502. - PubMed
-
- Hasty P., Campisi J., Hoeijmakers J., van Steeg H., Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–1359. - PubMed
-
- Goldman R.D., Shumaker D.K., Erdos M.R., Eriksson M., Goldman A.E., Gordon L.B., Gruenbaum Y., Khuon S., Mendez M., Varga R., Collins F.S. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 2004;101:8963–8968. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

