Comparative studies on structure of the floral nectaries and the abundance of nectar production of Prunus laurocerasus L
- PMID: 31312908
- PMCID: PMC6820602
- DOI: 10.1007/s00709-019-01412-z
Comparative studies on structure of the floral nectaries and the abundance of nectar production of Prunus laurocerasus L
Abstract
There is very scanty information concerning the floral nectary structure and nectar secretion in Prunus laurocerasus L. Therefore, the aim of the study was to determine the micromorphology, anatomy and ultrastructure of nectaries; the abundance of nectar production; and the quantitative and qualitative composition of sugars contained in the nectar of two P. laurocerasus cultivars: 'Schipkaensis' and 'Zabeliana'. The nectary structure was studied using light, fluorescence, scanning and transmission electron microscopy techniques. The nectar sugars were analysed with HPLC. The 'Schipkaensis' had longer inflorescences with a larger number of flowers and a longer perianth than 'Zabeliana'. The micromorphological structure of the nectaries in 'Schipkaensis' exhibited denser (approx. 39%) and larger (approx. 50%) stomata and thicker (approx. 13%) cuticular striae forming wider bands (approx. 26%) than in 'Zabeliana'. The results provide new data on the micromorphology, anatomy and ultrastructure of these floral nectaries. Nectary cuticle ornamentation as well as the size, type and density of stomata and stomatal complex topography can have a diagnostic value in Prunus. The nectar sugar weight indicates a significant apicultural value of the cherry laurel, especially in the case of 'Schipkaensis'. Cherry laurel is an entomophilous species recommended for cultivation in nectariferous zones and insect pollinator refuges; however, climatic conditions eliminating the invasiveness of these plants should be considered.
Keywords: Anatomy; Cherry laurel; Floral biology; Micromorphology; Sugar components; Ultrastructure.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





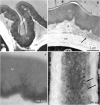


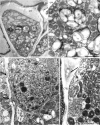


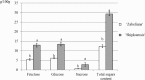
References
-
- Bernardello GA. A systematic survey of floral nectaries. In: Nicolson M, Nepi E, Pacini E, editors. Nectaries and nectar. Dordrecht: Springer; 2007. pp. 19–127.
-
- Bogdanov S, Martin P, Lüllmann C, Borneck R, Flamini Ch, Morlot MCh, Heretier J, Vorwohl G, Russmann H, Persano-Oddo L, Sabatini AG, Marcazzan GL, Marioleas P, Tsigouri K, Kerkvliet J, Ortiz A, Ivanov T (1997) Harmonised methods of the European honey commission. Apidologie Spec Iss 28:1–59
-
- Bordács MM, Botz L, Orosz-Kovács Z, Kerek MM. The composition of nectar in apricot cultivars. Acta Hortic. 1995;384:367–371. doi: 10.17660/ActaHortic.1995.384.58. - DOI
-
- Bukovics P, Orosz-Kovács Z, Szabó LG, Farkas Á, Bubán T. Composition of floral nectar and its seasonal variability in sour cherry cultivars. Acta Bot Hungar. 2003;45:259–271. doi: 10.1556/ABot.45.2003.3-4.2. - DOI
-
- Cameron RW, Taylor JE, Emmett NR. What’s ‘cool’ in the world of green façades? How plant choice influences the cooling properties of green walls. Build Environ. 2014;73:198–207. doi: 10.1016/j.buildenv.2013.12.005. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

