Diffuse smoking-related lung diseases: insights from a radiologic-pathologic correlation
- PMID: 31312909
- PMCID: PMC6635572
- DOI: 10.1186/s13244-019-0765-z
Diffuse smoking-related lung diseases: insights from a radiologic-pathologic correlation
Abstract
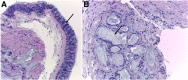
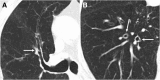
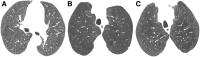
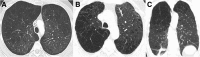
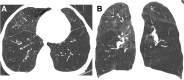
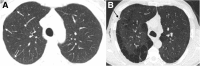
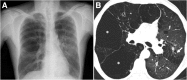
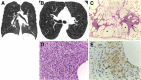
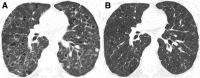
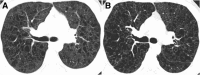
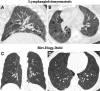
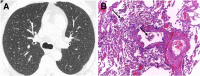
Cigarettes are well-recognized risk factors responsible for the emergence of a variety of pathologic conditions affecting both the airways and the lungs. Smoking-related lung diseases can be classified as chronic obstructive pulmonary disease (COPD) and several types of interstitial diseases, such as pulmonary Langerhans cell histiocytosis, bronchiolitis, desquamative interstitial pneumonitis, acute eosinophilic pneumonia, and interstitial fibrosing lung diseases. The evidence of combined lower lung fibrosis and predominant upper lung emphysema is renowned as a distinct clinical entity, named combined pulmonary fibrosis and emphysema. Although computerized tomography permits an adequate classification and distinction of these diseases, the clinical, imaging, and histological features often overlap and coexist in a single patient. Therefore, a combined radiologic and pathologic approach, in the appropriate clinical setting, is useful for best comprehension and distinction of these entities. Our goals are to describe the imaging features in smoking-related lung diseases and how the pathological manifestations translate on high-resolution computerized tomography.
Keywords: Bronchitis; Emphysema; Fibrosis; Interstitial lung diseases; Smoking.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



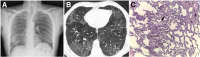
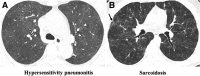
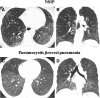
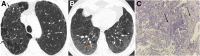
References
Publication types
LinkOut - more resources
Full Text Sources

