Long-term neurocognitive outcome is not worsened by of the use of venovenous ECMO in severe ARDS patients
- PMID: 31312911
- PMCID: PMC6635548
- DOI: 10.1186/s13613-019-0556-1
Long-term neurocognitive outcome is not worsened by of the use of venovenous ECMO in severe ARDS patients
Abstract
Background: Venovenous extracorporeal membrane oxygenation (VV-ECMO) is associated with a significant morbidity. There is the need to investigate long-term cognitive outcome among ARDS survivors treated with VV-ECMO. We aimed to compare the prevalence of long-term cognitive dysfunction and neuropsychological impairment using a highly specific test in severe ARDS survivors treated or not treated with VV-ECMO.
Methods: Severe ARDS survivors treated between 2011 and 2017 in an ECMO Regional Referral Center were prospectively evaluated 2 years after their ICU discharge. Patients underwent an in-person interview and examination. The primary outcome was cognitive function, assessed by the Wechsler Adult Intelligence Scale 4th edition (WAIS-IV). Secondary outcomes included anxiety, depression, post-traumatic stress disorder (PTSD) and quality-of-life.
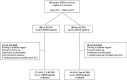
Results: We investigated 40 consecutive patients surviving from severe ARDS treated (N = 22) or not (N = 18) with VV-ECMO at a median [interquartile range] of 20 [17-22] and 22 [18-23] months after ICU discharge, respectively. Regarding the main outcome, cognitive function was below normal ranges in 12 (55%) ECMO patients and 10 (56%) non-ECMO patients (p = 0.95). Eight (36%) ECMO patients had moderate-to-severe depressive symptoms as compared with 7 (39%) non-ECMO patients (p = 0.87). Twelve (55%) ECMO patients and eight (44%) non-ECMO patients had moderate-to-severe anxiety symptoms (p = 0.53). Seven (33%) ECMO patients and eight (44%) non-ECMO patients presented a PTSD (p = 0.48). Health-related quality of life did not differ between the two groups.
Conclusions: Using the WAIS-IV, VV-ECMO treatment does not appear to worsen long-term cognitive and neuropsychological outcomes in severe ARDS patients.
Keywords: Cognitive impairment; Long-term cognitive and psychiatric morbidity; Long-term neuropsychological outcome; PTSD; WAIS-IV.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Extracorporeal Life Support Organization. Registry report: international summary January 2019. https://www.elso.org. Accessed 14 July 2019.
-
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. The Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. - DOI - PubMed
LinkOut - more resources
Full Text Sources