Brain-based ranking of cognitive domains to predict schizophrenia
- PMID: 31313451
- PMCID: PMC6865423
- DOI: 10.1002/hbm.24716
Brain-based ranking of cognitive domains to predict schizophrenia
Abstract
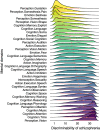
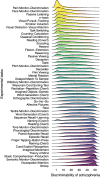
Schizophrenia is a devastating brain disorder that disturbs sensory perception, motor action, and abstract thought. Its clinical phenotype implies dysfunction of various mental domains, which has motivated a series of theories regarding the underlying pathophysiology. Aiming at a predictive benchmark of a catalog of cognitive functions, we developed a data-driven machine-learning strategy and provide a proof of principle in a multisite clinical dataset (n = 324). Existing neuroscientific knowledge on diverse cognitive domains was first condensed into neurotopographical maps. We then examined how the ensuing meta-analytic cognitive priors can distinguish patients and controls using brain morphology and intrinsic functional connectivity. Some affected cognitive domains supported well-studied directions of research on auditory evaluation and social cognition. However, rarely suspected cognitive domains also emerged as disease relevant, including self-oriented processing of bodily sensations in gustation and pain. Such algorithmic charting of the cognitive landscape can be used to make targeted recommendations for future mental health research.
Keywords: BrainMap database; coordinate-based meta-analysis; ontology of the mind; pattern recognition; predictive analytics; statistical learning.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no competing conflict of interest.
Figures





References
-
- Ansoleaga, B. , Garcia‐Esparcia, P. , Pinacho, R. , Haro, J. M. , Ramos, B. , & Ferrer, I. (2015). Decrease in olfactory and taste receptor expression in the dorsolateral prefrontal cortex in chronic schizophrenia. Journal of Psychiatric Research, 60, 109–116. 10.1016/j.jpsychires.2014.09.012 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

