Intrinsically Distinct Role of Neutrophil Extracellular Trap Formation in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Compared to Systemic Lupus Erythematosus
- PMID: 31313503
- PMCID: PMC7384043
- DOI: 10.1002/art.41047
Intrinsically Distinct Role of Neutrophil Extracellular Trap Formation in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Compared to Systemic Lupus Erythematosus
Abstract
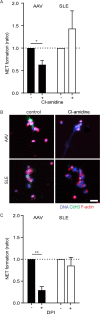
Objective: Different studies have demonstrated that neutrophil extracellular traps (NETs) may be involved in the pathophysiology of both antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) and systemic lupus erythematosus (SLE). AAV and SLE are clinically and pathologically divergent autoimmune diseases with different autoantibodies. However, the respective autoantigens recognized in AAV and SLE have been shown to be an intricate part of NETs. This study aimed to examine whether the mechanisms of NET formation and the composition of NETs are distinct between AAV and SLE.
Methods: To investigate this hypothesis, healthy neutrophils were stimulated with serum from patients with AAV (n = 80) and patients with SLE (n = 59), and the mechanisms of NET formation and NET composition were compared.
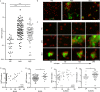
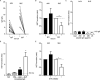
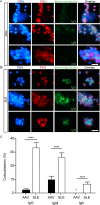
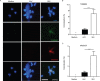
Results: Both patients with AAV and patients with SLE had excessive NET formation, which correlated with the extent of disease activity (in AAV r = 0.5, P < 0.0001; in SLE r = 0.35, P < 0.01). Lytic NET formation over several hours was observed in patients with AAV, as compared to rapid (within minutes), non-lytic NET formation coinciding with clustering of neutrophils in patients with SLE. AAV-induced NET formation was triggered independent of IgG ANCAs, whereas SLE immune complexes (ICx) induced NET formation through Fcγ receptor signaling. AAV-induced NET formation was dependent on reactive oxygen species and peptidyl arginine deaminases, and AAV-induced NETs were enriched for citrullinated histones (mean ± SEM 23 ± 2%). In contrast, SLE-induced NETs had immunogenic properties, including binding with high mobility group box chromosomal protein 1 (mean ± SEM 30 ± 3%) and enrichment for oxidized mitochondrial DNA, and were involved in ICx formation.
Conclusion: The morphologic features, kinetics, induction pathways, and composition of excessive NET formation are all intrinsically distinct in AAV compared to SLE. Recognizing the diversity of NET formation between AAV and SLE provides a better understanding of the pathophysiologic role of NETs in these different autoimmune diseases.
© 2019 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures

References
-
- D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet 2007;369:587–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

