Comparison of Major Adverse Cardiac Events Between Instantaneous Wave-Free Ratio and Fractional Flow Reserve-Guided Strategy in Patients With or Without Type 2 Diabetes: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 31314045
- PMCID: PMC6646975
- DOI: 10.1001/jamacardio.2019.2298
Comparison of Major Adverse Cardiac Events Between Instantaneous Wave-Free Ratio and Fractional Flow Reserve-Guided Strategy in Patients With or Without Type 2 Diabetes: A Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: Invasive physiologic indices such as fractional flow reserve (FFR) and instantaneous wave-free ratio (iFR) are used in clinical practice. Nevertheless, comparative prognostic outcomes of iFR-guided and FFR-guided treatment in patients with type 2 diabetes have not yet been fully investigated.
Objective: To compare 1-year clinical outcomes of iFR-guided or FFR-guided treatment in patients with and without diabetes in the Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularization (DEFINE-FLAIR) trial.
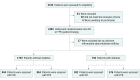
Design, setting, and participants: The DEFINE-FLAIR trial is a multicenter, international, randomized, double-blinded trial that randomly assigned 2492 patients in a 1:1 ratio to undergo either iFR-guided or FFR-guided coronary revascularization. Patients were eligible for trial inclusion if they had intermediate coronary artery disease (40%-70% diameter stenosis) in at least 1 native coronary artery. Data were analyzed between January 2014 and December 2015.
Interventions: According to the study protocol, iFR of 0.89 or less and FFR of 0.80 or less were used as criteria for revascularization. When iFR or FFR was higher than the prespecified threshold, revascularization was deferred.
Main outcomes and measures: The primary end point was major adverse cardiac events (MACE), defined as the composite of all-cause death, nonfatal myocardial infarction, or unplanned revascularization at 1 year. The incidence of MACE was compared according to the presence of diabetes in iFR-guided and FFR-guided groups.
Results: Among the total trial population (2492 patients), 758 patients (30.4%) had diabetes. Mean age of the patients was 66 years, 76% were men (1868 of 2465), and 80% of patients presented with stable angina (1983 of 2465). In the nondiabetes population (68.5%; 1707 patients), iFR guidance was associated with a significantly higher rate of deferral of revascularization than the FFR-guided group (56.5% [n = 477 of 844] vs 46.6% [n = 402 of 863]; P < .001). However, it was not different between the 2 groups in the diabetes population (42.1% [n = 161 of 382] vs 47.1% [n = 177 of 376]; P = .15). At 1 year, the diabetes population showed a significantly higher rate of MACE than the nondiabetes population (8.6% vs 5.6%; adjusted hazard ratio [HR], 1.88; 95% CI, 1.28-2.64; P < .001). However, there was no significant difference in MACE rates between iFR-guided and FFR-guided groups in both the diabetes (10.0% vs 7.2%; adjusted HR, 1.33; 95% CI, 0.78-2.25; P = .30) and nondiabetes population (4.7% vs 6.4%; HR, 0.83; 95% CI, 0.51-1.35; P = .45) (interaction P = .25).
Conclusions and relevance: The diabetes population showed significantly higher risk of MACE than the nondiabetes population, even with the iFR-guided or FFR-guided treatment. The iFR-guided and FFR-guided treatment showed comparable risk of MACE and provided equal safety in selecting revascularization target among patients with diabetes.
Trial registration: ClinicalTrials.gov identifier: NCT02053038.
Conflict of interest statement
Figures


References
-
- Levine GN, Bates ER, Blankenship JC, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions . 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):e44-e122. doi:10.1016/j.jacc.2011.08.007 - DOI - PubMed
-
- Windecker S, Kolh P, Alfonso F, et al. ; Authors/Task Force members . 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541-2619. doi:10.1093/eurheartj/ehu278 - DOI - PubMed

