Estradiol rapidly modulates excitatory synapse properties in a sex- and region-specific manner in rat nucleus accumbens core and caudate-putamen
- PMID: 31314648
- PMCID: PMC6766735
- DOI: 10.1152/jn.00264.2019
Estradiol rapidly modulates excitatory synapse properties in a sex- and region-specific manner in rat nucleus accumbens core and caudate-putamen
Abstract
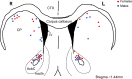
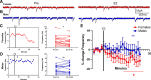
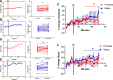
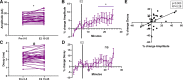
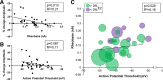
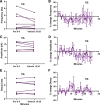
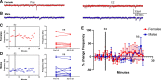
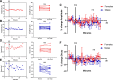
Estradiol acutely facilitates sex differences in striatum-dependent behaviors. However, little is understood regarding the underlying mechanism. In striatal regions in adult rodents, estrogen receptors feature exclusively extranuclear expression, suggesting that estradiol rapidly modulates striatal neurons. We tested the hypothesis that estradiol rapidly modulates excitatory synapse properties onto medium spiny neurons (MSNs) of two striatal regions, the nucleus accumbens core and caudate-putamen in adult female and male rats. We predicted there would be sex-specific differences in pre- and postsynaptic locus and sensitivity. We further analyzed whether MSN intrinsic properties are predictive of estrogen sensitivity. Estradiol exhibited sex-specific acute effects in the nucleus accumbens core: miniature excitatory postsynaptic current (mEPSC) frequency robustly decreased in response to estradiol in female MSNs, and mEPSC amplitude moderately increased in response to estradiol in both male and female MSNs. This increase in mEPSC amplitude is associated with MSNs featuring increased intrinsic excitability. No MSN intrinsic electrical property associated with changes in mEPSC frequency. Estradiol did not acutely modulate mEPSC properties in the caudate-putamen of either sex. This is the first demonstration of acute estradiol action on MSN excitatory synapse function. This demonstration of sex and striatal region-specific acute estradiol neuromodulation revises our understanding of sex hormone action on striatal physiology and resulting behaviors.NEW & NOTEWORTHY This study is the first to demonstrate rapid estradiol neuromodulation of glutamatergic signaling on medium spiny neurons (MSNs), the major output neuron of the striatum. These findings emphasize that sex is a significant biological variable both in MSN sensitivity to estradiol and in pre- and postsynaptic mechanisms of glutamatergic signaling. MSNs in different regions exhibit diverse responses to estradiol. Sex- and region-specific estradiol-induced changes to excitatory signaling on MSNs explain sex differences partially underlying striatum-mediated behaviors and diseases.
Keywords: caudate-putamen; estradiol; medium spiny neuron; miniature excitatory postsynaptic currents; nucleus accumbens; striatum.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
-
- Adams C, DeFazio RA, Christian CA, Milescu LS, Schnell S, Moenter SM. Changes in both neuron intrinsic properties and neurotransmission are needed to drive the increase in GnRH neuron firing rate during estradiol-positive feedback. J Neurosci 39: 2091–2101, 2019. doi: 10.1523/JNEUROSCI.2880-18.2019. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

