Dosing pole recommendations for lymphatic filariasis elimination: A height-weight quantile regression modeling approach
- PMID: 31314753
- PMCID: PMC6663033
- DOI: 10.1371/journal.pntd.0007541
Dosing pole recommendations for lymphatic filariasis elimination: A height-weight quantile regression modeling approach
Abstract
Background: The World Health Organization (WHO) currently recommends height or age-based dosing as alternatives to weight-based dosing for mass drug administration lymphatic filariasis (LF) elimination programs. The goals of our study were to compare these alternative dosing strategies to weight-based dosing and to develop and evaluate new height-based dosing pole scenarios.
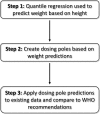
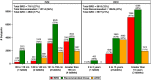
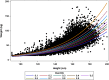
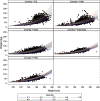
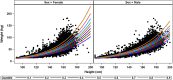
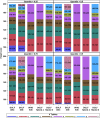
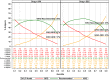
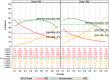
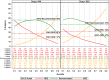
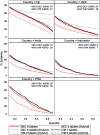
Methodology/principal findings: Age, height and weight data were collected from >26,000 individuals in five countries during a cluster randomized LF clinical trial. Weight-based dosing for diethylcarbamazine (DEC; 6 mg/kg) and ivermectin (IVM; 200 ug/kg) with tablet numbers derived from a table of weight intervals was treated as the "gold standard" for this study. Following WHO recommended age-based dosing of DEC and height-based dosing of IVM would have resulted in 32% and 27% of individuals receiving treatment doses below those recommended by weight-based dosing for DEC and IVM, respectively. Underdosing would have been especially common in adult males, who tend to have the highest LF prevalence in many endemic areas. We used a 3-step modeling approach to develop and evaluate new dosing pole cutoffs. First, we analyzed the clinical trial data using quantile regression to predict weight from height. We then used weight predictions to develop new dosing pole cutoff values. Finally, we compared different dosing pole cutoffs and age and height-based WHO dosing recommendations to weight-based dosing. We considered hundreds of scenarios including country- and sex-specific dosing poles. A simple dosing pole with a 6-tablet maximum for both DEC and IVM reduced the underdosing rate by 30% and 21%, respectively, and was nearly as effective as more complex pole combinations for reducing underdosing.
Conclusions/significance: Using a novel modeling approach, we developed a simple dosing pole that would markedly reduce underdosing for DEC and IVM in MDA programs compared to current WHO recommended height or age-based dosing.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
The effect of six rounds of single dose mass treatment with diethylcarbamazine or ivermectin on Wuchereria bancrofti infection and its implications for lymphatic filariasis elimination.Trop Med Int Health. 2002 Sep;7(9):767-74. doi: 10.1046/j.1365-3156.2002.00935.x. Trop Med Int Health. 2002. PMID: 12225508 Clinical Trial.
-
The impact of six rounds of single-dose mass administration of diethylcarbamazine or ivermectin on the transmission of Wuchereria bancrofti by Culex quinquefasciatus and its implications for lymphatic filariasis elimination programmes.Trop Med Int Health. 2003 Dec;8(12):1082-92. doi: 10.1046/j.1360-2276.2003.01138.x. Trop Med Int Health. 2003. PMID: 14641843 Clinical Trial.
-
Efficacy, Safety, and Pharmacokinetics of Coadministered Diethylcarbamazine, Albendazole, and Ivermectin for Treatment of Bancroftian Filariasis.Clin Infect Dis. 2016 Feb 1;62(3):334-341. doi: 10.1093/cid/civ882. Epub 2015 Oct 20. Clin Infect Dis. 2016. PMID: 26486704 Clinical Trial.
-
Adverse events following single dose treatment of lymphatic filariasis: Observations from a review of the literature.PLoS Negl Trop Dis. 2018 May 16;12(5):e0006454. doi: 10.1371/journal.pntd.0006454. eCollection 2018 May. PLoS Negl Trop Dis. 2018. PMID: 29768412 Free PMC article. Review.
-
Salt fortified with diethylcarbamazine (DEC) as an effective intervention for lymphatic filariasis, with lessons learned from salt iodization programmes.Parasitology. 2000;121 Suppl:S161-73. doi: 10.1017/s0031182000007150. Parasitology. 2000. PMID: 11386687 Review.
Cited by
-
A Roadmap for the Development of Ivermectin as a Complementary Malaria Vector Control Tool.Am J Trop Med Hyg. 2020 Feb;102(2s):3-24. doi: 10.4269/ajtmh.19-0620. Am J Trop Med Hyg. 2020. PMID: 31971144 Free PMC article.
-
Evaluation of Microfilaremic Individuals after Mass Drug Treatment with Ivermectin, Diethylcarbamazine, and Albendazole for Lymphatic Filariasis in Papua New Guinea.Am J Trop Med Hyg. 2025 Mar 25;112(6):1235-1239. doi: 10.4269/ajtmh.24-0382. Print 2025 Jun 4. Am J Trop Med Hyg. 2025. PMID: 40132219 Free PMC article.
-
Prevention of bacterial complications of scabies using mass drug administration: A population-based, before-after trial in Fiji, 2018-2020.Lancet Reg Health West Pac. 2022 Mar 22;22:100433. doi: 10.1016/j.lanwpc.2022.100433. eCollection 2022 May. Lancet Reg Health West Pac. 2022. PMID: 35345391 Free PMC article.
-
Use of a "tablet pole" for the administration of ivermectin for strongyloidiasis in a field study in Ecuador.Infect Dis Poverty. 2023 Jan 28;12(1):3. doi: 10.1186/s40249-023-01054-7. Infect Dis Poverty. 2023. PMID: 36709311 Free PMC article.
-
Safety and efficacy of co-administered diethylcarbamazine, albendazole and ivermectin during mass drug administration for lymphatic filariasis in Haiti: Results from a two-armed, open-label, cluster-randomized, community study.PLoS Negl Trop Dis. 2020 Jun 8;14(6):e0008298. doi: 10.1371/journal.pntd.0008298. eCollection 2020 Jun. PLoS Negl Trop Dis. 2020. PMID: 32511226 Free PMC article. Clinical Trial.
References
-
- WHO. Global programme to eliminate lymphatic filariasis: progress report, 2017. Weekly epidemiological record. 2018;93(44):589–604.
-
- WHO. Progress report 2000–2009 and strategic plan 2010–2020 of the global programme to eliminate lymphatic filariasis: halfway towards elminating lymphatic filariasis. 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

