Nutrient-enriched formula versus standard formula for preterm infants
- PMID: 31314903
- PMCID: PMC6636703
- DOI: 10.1002/14651858.CD004204.pub3
Nutrient-enriched formula versus standard formula for preterm infants
Abstract
Background: Preterm infants may accumulate nutrient deficits leading to extrauterine growth restriction. Feeding preterm infants with nutrient-enriched rather than standard formula might increase nutrient accretion and growth rates and might improve neurodevelopmental outcomes.
Objectives: To compare the effects of feeding with nutrient-enriched formula versus standard formula on growth and development of preterm infants.
Search methods: We used the Cochrane Neonatal standard search strategy. This included electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 11), MEDLINE, Embase, and the Cumulative Index to Nursing and Allied Health Literature (until November 2018), as well as conference proceedings, previous reviews, and clinical trials databases.
Selection criteria: Randomised and quasi-randomised controlled trials that compared feeding preterm infants with nutrient-enriched formula (protein and energy plus minerals, vitamins, or other nutrients) versus standard formula.
Data collection and analysis: We extracted data using the Cochrane Neonatal standard methods. Two review authors separately evaluated trial quality and extracted and synthesised data using risk ratios (RRs), risk differences, and mean differences (MDs). We assessed certainty of evidence at the outcome level using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methods.
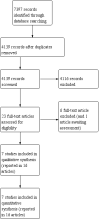
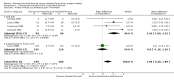
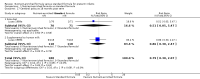
Main results: We identified seven trials in which a total of 590 preterm infants participated. Most participants were clinically stable preterm infants of birth weight less than 1850 g. Few participants were extremely preterm, extremely low birth weight, or growth restricted at birth. Trials were conducted more than 30 years ago, were formula industry funded, and were small with methodological weaknesses (including lack of masking) that might bias effect estimates. Meta-analyses of in-hospital growth parameters were limited by statistical heterogeneity. There is no evidence of an effect on time to regain birth weight (MD -1.48 days, 95% confidence interval (CI) -4.73 to 1.77) and low-certainty evidence suggests that feeding with nutrient-enriched formula increases in-hospital rates of weight gain (MD 2.43 g/kg/d, 95% CI 1.60 to 3.26) and head circumference growth (MD 1.04 mm/week, 95% CI 0.18 to 1.89). Meta-analysis did not show an effect on the average rate of length gain (MD 0.22 mm/week, 95% CI -0.70 to 1.13). Fewer data are available for growth and developmental outcomes assessed beyond infancy, and these do not show consistent effects of nutrient-enriched formula feeding. Data from two trials did not show an effect on Bayley Mental Development Index scores at 18 months post term (MD 2.87, 95% CI -1.38 to 7.12; moderate-certainty evidence). Infants who received nutrient-enriched formula had higher Bayley Psychomotor Development Index scores at 18 months post term (MD 6.56. 95% CI 2.87 to 10.26; low-certainty evidence), but no evidence suggested an effect on cerebral palsy (typical RR 0.79, 95% CI 0.30 to 2.07; 2 studies, 377 infants). Available data did not indicate any other benefits or harms and provided low-certainty evidence about the effect of nutrient-enriched formula feeding on the risk of necrotising enterocolitis in preterm infants (typical RR 0.72, 95% CI 0.41 to 1.25; 3 studies, 489 infants).
Authors' conclusions: Available trial data show that feeding preterm infants nutrient-enriched (compared with standard) formulas has only modest effects on growth rates during their initial hospital admission. No evidence suggests effects on long-term growth or development. The GRADE assessment indicates that the certainty of this evidence is low, and that these findings should be interpreted and applied with caution. Further randomised trials would be needed to resolve this uncertainty.
Conflict of interest statement
NDE declares the following: receiving a research grant award for an RCT of breast milk products by Prolacta Bioscience, 2017; receiving a grant from Danone Early Life Nutrition to support a study on feeding in late and moderately preterm infants, 2018; receiving a grant from Nestle Nutrition for transcriptomic analyses of gut tissue, 2016; lectures with Wyeth Nutrition in 2017, Nestle Nutrition Institute in 2017 and 2018, Philipps in 2017, and Fresenius, 2017.
VW has no conflicts of interest.
JB has no conflicts of interest.
LA has no conflicts of interest.
WM has no conflicts of interest.
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, distinct from the Gerber Products Company. The grantor has no input on the content of the review nor on the editorial process.
To maintain the utmost editorial independence for this Cochrane Review, an editor outside of the Cochrane Neonatal core editorial team who is not receiving any financial remuneration from the grant, Eugene Dempsey, was the Sign‐off Editor for this review. Additionally, a Senior Editor from the Cochrane Children and Families Network, Robert Boyle, assessed and signed off on this Cochrane Review.
Figures

































Update of
References
References to studies included in this review
Kashyap 1986 {published data only}
-
- Kashyap S, Forsyth M, Zucker C, Ramakrishnan R, Dell RB, Heird WC. Effects of varying protein and energy intakes on growth and metabolic response in low birth weight infants. Journal of Pediatrics 1986;108(6):955‐63. [PUBMED: 3712165] - PubMed
Kulkarni 1984 {published data only}
-
- Kulkarni PB, Dorand RD, Bridger WM, Payne JH, Montiel DC, Hill JG. Rickets in premature infants fed different formulas. Southern Medical Journal 1984;77(1):13‐6, 20. [PUBMED: 6364370] - PubMed
Lucas 1989a {published data only}
-
- Bishop NJ, Dahlenburg SL, Fewtrell MS, Morley R, Lucas A. Early diet of preterm infants and bone mineralization at age five years. Acta Paediatrica 1996;85(2):230‐6. [PUBMED: 8640056] - PubMed
-
- Fewtrell MS, Prentice A, Jones SC, Bishop NJ, Stirling D, Buffenstein R, et al. Bone mineralization and turnover in preterm infants at 8‐12 years of age: the effect of early diet. Journal of Bone and Mineral Research 1999;14(5):810‐20. [DOI: 10.1359/jbmr.1999.14.5.810; PUBMED: 10320530] - DOI - PubMed
Lucas 1989b {published data only}
-
- Bishop NJ, Dahlenburg SL, Fewtrell MS, Morley R, Lucas A. Early diet of preterm infants and bone mineralization at age five years. Acta Paediatrica 1996;85(2):230‐6. [PUBMED: 8640056] - PubMed
-
- Fewtrell MS, Prentice A, Jones SC, Bishop NJ, Stirling D, Buffenstein R, et al. Bone mineralization and turnover in preterm infants at 8‐12 years of age: the effect of early diet. Journal of Bone and Mineral Research 1999;14(5):810‐20. [DOI: 10.1359/jbmr.1999.14.5.810; PUBMED: 10320530] - DOI - PubMed
-
- Fewtrell MS, Williams JE, Singhal A, Murgatroyd PR, Fuller N, Lucas A. Early diet and peak bone mass: 20 year follow‐up of a randomized trial of early diet in infants born preterm. Bone 2009;45(1):142‐9. [PUBMED: 19306955] - PubMed
Siripoonya 1989 {published data only}
-
- Siripoonya P, Sasivimolkul V, Tejavej A, Hotrakitya S, Tontisirin K. Clinical trial of special premature formula for low‐birth‐weight infants. Journal of the Medical Association of Thailand 1989;72 Suppl 1:61‐5. [PUBMED: 2659718] - PubMed
Thom 1984 {published data only}
-
- Thom JC, Jong G, Kotze TJ. Clinical trial of a milk formula for infants of low birth weight. South African Medical Journal 1984;65(4):125‐7. [PUBMED: 6364395] - PubMed
Yesilipek 1992 {published data only}
-
- Yesilipek MA. Standard and low birth weight formulas compared for effects on growth of preterm infants. Turkish Journal of Pediatrics 1992;34(1):31‐6. [PUBMED: 1509527] - PubMed
References to studies excluded from this review
Atkinson 1981 {published data only}
-
- Atkinson SA, Bryan MH, Anderson GH. Human milk feeding in premature infants: protein, fat, and carbohydrate balances in the first two weeks of life. Journal of Pediatrics 1981;99(4):617‐24. [PUBMED: 7277107] - PubMed
Duman 2000 {published data only}
-
- Duman N, Utkutan S, Ozkan H, Ozdogan S. Are the stool characteristics of preterm infants affected by infant formulas?. Turkish Journal of Pediatrics 2000;42(2):138‐44. [PUBMED: 10936980] - PubMed
Haque 1987 {published data only}
-
- Haque KN, Bashir O, Lanbourne A, Mullen J. Trial of a new high density milk formula (Prematalac) in Saudi preterm and low birth weight infants. Saudi Medical Journal 1987;8:77‐86.
Hering 1987 {published data only}
-
- Hering E, Vaisman S, Beca JP. Evaluation of a modified milk formula in low birth weight neonates [Evaluacion de una formula de leche modificada en recien nacidos de bajo peso]. Revista Chilena de Pediatria 1987;58(3):197‐202. [PUBMED: 3454457] - PubMed
Pridham 1999 {published data only}
-
- Pridham K, Kosorok MR, Greer F, Carey P, Kayata S, Sondel S. The effects of prescribed versus ad libitum feedings and formula caloric density on premature infant dietary intake and weight gain. Nursing Research 1999;48(2):86‐93. [PUBMED: 10190835] - PubMed
Yin 2004 {published data only}
-
- Lin YF, Hsieh KS. Chen YY. Nutrient‐enriched versus standard term formula feeding in disproportionately small for gestational age infants. Clinical Neonatology 2004;11:36‐39.
References to studies awaiting assessment
Additional references
AAP 2012
Agostoni 2010
-
- Agostoni C, Buonocore G, Carnielli VP, Curtis M, Darmaun D, Decsi T, et al. ESPGHAN Committee on Nutrition. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):85‐91. [DOI: 10.1097/MPG.0b013e3181adaee0; PUBMED: 19881390] - DOI - PubMed
Bracewell 2008
-
- Bracewell MA, Hennessy EM, Wolke D, Marlow N. The EPICure study: growth and blood pressure at 6 years of age following extremely preterm birth. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(2):F108‐14. [PUBMED: 17660214] - PubMed
Brown 2016
Clark 2003
-
- Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003;111(5 Pt 1):986‐90. - PubMed
Cleminson 2015
Cooke 2003
Doyle 2004
Duffy 2016
Dusick 2003
-
- Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up?. Seminars in Perinatology 2003;27(4):302‐10. [PUBMED: 14510321] - PubMed
Embleton 2001
-
- Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants?. Pediatrics 2001;107(2):270‐3. - PubMed
Embleton 2013
-
- Embleton ND. Early nutrition and later outcomes in preterm infants. World Review of Nutrition and Dietetics 2013;106:26‐32. [PUBMED: 23428677] - PubMed
Embleton 2017
Euser 2005
-
- Euser AM, Finken MJ, Keijzer‐Veen MG, Hille ET, Wit JM, Dekker FW, Dutch POPS‐19 Collaborative Study Group. Associations between prenatal and infancy weight gain and BMI, fat mass, and fat distribution in young adulthood: a prospective cohort study in males and females born very preterm. American Journal of Clinical Nutrition 2005;81(2):480‐7. [PUBMED: 15699238] - PubMed
Euser 2008
-
- Euser AM, Wit CC, Finken MJ, Rijken M, Wit JM. Growth of preterm born children. Hormone Research 2008;70(6):319‐28. [PUBMED: 18953169] - PubMed
Farooqi 2006
-
- Farooqi A, Hägglöf B, Sedin G, Gothefors L, Serenius F. Growth in 10‐ to 12‐year‐old children born at 23 to 25 weeks' gestation in the 1990s: a Swedish national prospective follow‐up study. Pediatrics 2006;118(5):e1452‐65. [PUBMED: 17079546] - PubMed
Fewtrell 2011
-
- Fewtrell M. Early nutritional predictors of long‐term bone health in preterm infants. Current Opinion in Clinical Nutrition and Metabolic Care 2011;14(3):297‐301. [PUBMED: 21378555] - PubMed
Ford 2000
-
- Ford GW, Doyle LW, Davis NM, Callanan C. Very low birth weight and growth into adolescence. Archives of Pediatrics and Adolescent Medicine 2000;154(8):778‐84. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 7 August 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Hack 1991
-
- Hack M, Breslau N, Weissman B, Aram D, Klein N, Borawski E. Effect of very low birth weight and subnormal head size on cognitive abilities at school age. New England Journal of Medicine 1991;325(4):231–7. - PubMed
Hancock 1984
-
- Hancock PJ, Bancalari E. Gastric motility in premature infants fed two different formulas. Journal of Pediatric Gastroenterology and Nutrition 1984;3(5):696‐9. - PubMed
Hay 2017
-
- Hay WW Jr, Hendrickson KC. Preterm formula use in the preterm very low birth weight infant. Seminars in Fetal & Neonatal Medicine 2017;22(1):15‐22. [PUBMED: 27595621] - PubMed
Higgins 2017
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Horbar 2015
-
- Horbar JD, Ehrenkranz RA, Badger GJ, Edwards EM, Morrow KA, Soll RF, et al. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000‐2013. Pediatrics 2015;136(1):e84‐92. [PUBMED: 26101360] - PubMed
Imdad 2013
Klein 2002
-
- Klein CJ. Nutrient requirements for preterm infant formulas. Journal of Nutrition 2002;132(6):1395S‐577S. - PubMed
Kliegman 1987
Klingenberg 2012
Lapillonne 2013
-
- Lapillonne A, Griffin IJ. Feeding preterm infants today for later metabolic and cardiovascular outcomes. Journal of Pediatrics 2013;162(3 Suppl):S7‐16. [PUBMED: 23445851] - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6. Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Leppänen 2014
-
- Leppänen M, Lapinleimu H, Lind A, Matomäki J, Lehtonen L, Haataja L, et al. PIPARI Study Group. Antenatal and postnatal growth and 5‐year cognitive outcome in very preterm infants. Pediatrics 2014;133(1):63‐70. [PUBMED: 24344103] - PubMed
Luttikhuizen 2013
-
- Luttikhuizen dos Santos ES, Kieviet JF, Konigs M, Elburg RM, Oosterlaan J. Predictive value of the Bayley scales of infant development on development of very preterm/very low birth weight children: a meta‐analysis. Early Human Development 2013;89(7):487‐96. [DOI: 10.1016/j.earlhumdev.2013.03.008; PUBMED: 23597678] - DOI - PubMed
Ouzzani 2016
Ramani 2013
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shulhan 2017
Siegel 1984
-
- Siegel M, Lebenthal E, Krantz B. Effect of caloric density on gastric emptying in premature infants. Journal of Pediatrics 1984;104(1):118‐22. - PubMed
Trebar 2007
-
- Trebar B, Traunecker R, Selbmann HK, Ranke MB. Growth during the first two years predicts pre‐school height in children born with very low birth weight (VLBW): results of a study of 1,320 children in Germany. Pediatric Research 2007;62(2):209‐14. [PUBMED: 17597641] - PubMed
Tsang 1993
-
- Tsang RC, Lucas A, Uauy R, Zlotkin S. Nutritional Needs of the Newborn Infant: Scientific Basis and Practical Guidelines. Pawling, New York: Caduceus Medical Publishers, 1993.
Veritas Health Innovation [Computer program]
-
- Veritas Health Innovation. Covidence. Melbourne, Australia: Veritas Health Innovation, accessed prior to 13 June 2019.
WHO 2018
-
- WHO, UNICEF, IBFAN. Marketing of breast‐milk substitutes: national implementation of the international code, status report. Geneva: World Health Organization, 2018; Vol. Licence: CC BY‐NC‐SA 3.0 IGO.
References to other published versions of this review
Simmer 2003
-
- Simmer K, Askie LM 2003, Issue 2. Art. No.: CD004204. DOI: 10.1002/14651858.CD004204. Preterm formula milk versus term formula milk for feeding preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004204] - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

