Universal Testing, Expanded Treatment, and Incidence of HIV Infection in Botswana
- PMID: 31314967
- PMCID: PMC6800102
- DOI: 10.1056/NEJMoa1812281
Universal Testing, Expanded Treatment, and Incidence of HIV Infection in Botswana
Abstract
Background: The feasibility of reducing the population-level incidence of human immunodeficiency virus (HIV) infection by increasing community coverage of antiretroviral therapy (ART) and male circumcision is unknown.
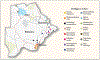
Methods: We conducted a pair-matched, community-randomized trial in 30 rural or periurban communities in Botswana from 2013 to 2018. Participants in 15 villages in the intervention group received HIV testing and counseling, linkage to care, ART (started at a higher CD4 count than in standard care), and increased access to male circumcision services. The standard-care group also consisted of 15 villages. Universal ART became available in both groups in mid-2016. We enrolled a random sample of participants from approximately 20% of households in each community and measured the incidence of HIV infection through testing performed approximately once per year. The prespecified primary analysis was a permutation test of HIV incidence ratios. Pair-stratified Cox models were used to calculate 95% confidence intervals.
Results: Of 12,610 enrollees (81% of eligible household members), 29% were HIV-positive. Of the 8974 HIV-negative persons (4487 per group), 95% were retested for HIV infection over a median of 29 months. A total of 57 participants in the intervention group and 90 participants in the standard-care group acquired HIV infection (annualized HIV incidence, 0.59% and 0.92%, respectively). The unadjusted HIV incidence ratio in the intervention group as compared with the standard-care group was 0.69 (P = 0.09) by permutation test (95% confidence interval [CI], 0.46 to 0.90 by pair-stratified Cox model). An end-of-trial survey in six communities (three per group) showed a significantly greater increase in the percentage of HIV-positive participants with an HIV-1 RNA level of 400 copies per milliliter or less in the intervention group (18 percentage points, from 70% to 88%) than in the standard-care group (8 percentage points, from 75% to 83%) (relative risk, 1.12; 95% CI, 1.09 to 1.16). The percentage of men who underwent circumcision increased by 10 percentage points in the intervention group and 2 percentage points in the standard-care group (relative risk, 1.26; 95% CI, 1.17 to 1.35).
Conclusions: Expanded HIV testing, linkage to care, and ART coverage were associated with increased population viral suppression. (Funded by the President's Emergency Plan for AIDS Relief and others; Ya Tsie ClinicalTrials.gov number, NCT01965470.).
Copyright © 2019 Massachusetts Medical Society.
Figures

Comment in
-
HIV-1 Epidemic Control - Insights from Test-and-Treat Trials.N Engl J Med. 2019 Jul 18;381(3):286-288. doi: 10.1056/NEJMe1907279. N Engl J Med. 2019. PMID: 31314975 No abstract available.
-
Universal Testing and Treatment for HIV Infection in Botswana.N Engl J Med. 2019 Nov 28;381(22):2180. doi: 10.1056/NEJMc1911065. N Engl J Med. 2019. PMID: 31774970 Free PMC article. No abstract available.
References
-
- Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016;316:171–81. - PubMed
-
- Bavinton BR, Pinto AN, Phanuphak N, et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV 2018;5(8):e438–e447. - PubMed
-
- Rodger A, Cambiano V, Bruun T, et al. HIV transmission risk through condomless sex in gay couples with suppressive ART: the PARTNER2 Study extended results in gay men. Presented at the 22nd International AIDS Conference, Amsterdam, July 23–27, 2018 (slide presentation).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K23 AI091434/AI/NIAID NIH HHS/United States
- R01 AI127271/AI/NIAID NIH HHS/United States
- U01 GH000447/GH/CGH CDC HHS/United States
- 107752/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- D43 TW009610/TW/FIC NIH HHS/United States
- R37 AI051164/AI/NIAID NIH HHS/United States
- K23 HD070774/HD/NICHD NIH HHS/United States
- D43 TW000004/TW/FIC NIH HHS/United States
- R01 CA236546/CA/NCI NIH HHS/United States
- R01 AI104459/AI/NIAID NIH HHS/United States
- R01 CA222147/CA/NCI NIH HHS/United States
- K24 AI131928/AI/NIAID NIH HHS/United States
- U2G GH001911/GH/CGH CDC HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
