Inflammation and Cancer: Triggers, Mechanisms, and Consequences
- PMID: 31315034
- PMCID: PMC6831096
- DOI: 10.1016/j.immuni.2019.06.025
Inflammation and Cancer: Triggers, Mechanisms, and Consequences
Abstract
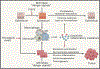
Inflammation predisposes to the development of cancer and promotes all stages of tumorigenesis. Cancer cells, as well as surrounding stromal and inflammatory cells, engage in well-orchestrated reciprocal interactions to form an inflammatory tumor microenvironment (TME). Cells within the TME are highly plastic, continuously changing their phenotypic and functional characteristics. Here, we review the origins of inflammation in tumors, and the mechanisms whereby inflammation drives tumor initiation, growth, progression, and metastasis. We discuss how tumor-promoting inflammation closely resembles inflammatory processes typically found during development, immunity, maintenance of tissue homeostasis, or tissue repair and illuminate the distinctions between tissue-protective and pro-tumorigenic inflammation, including spatiotemporal considerations. Defining the cornerstone rules of engagement governing molecular and cellular mechanisms of tumor-promoting inflammation will be essential for further development of anti-cancer therapies.
Keywords: cancer; cell plasticity; cytokine; inflammation; mechanisms; metastasis; tumor microenvironment; tumor progression.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Akkari L, Gocheva V, Kester JC, Hunter KE, Quick ML, Sevenich L, Wang HW, Peters C, Tang LH, Klimstra DS, et al. (2014). Distinct functions of macrophage-derived and cancer cell-derived cathepsin Z combine to promote tumor malignancy via interactions with the extracellular matrix. Genes Dev 28, 2134–2150. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

