Spinal Neuropeptide Y1 Receptor-Expressing Neurons Form an Essential Excitatory Pathway for Mechanical Itch
- PMID: 31315043
- PMCID: PMC6709688
- DOI: 10.1016/j.celrep.2019.06.033
Spinal Neuropeptide Y1 Receptor-Expressing Neurons Form an Essential Excitatory Pathway for Mechanical Itch
Abstract
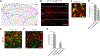
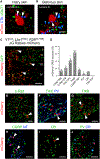
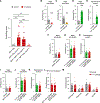
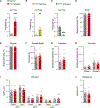
Acute itch can be generated by either chemical or mechanical stimuli, which activate separate pathways in the periphery and spinal cord. While substantial progress has been made in mapping the transmission pathway for chemical itch, the central pathway for mechanical itch remains obscure. Using complementary genetic and pharmacological manipulations, we show that excitatory neurons marked by the expression of the neuropeptide Y1 receptor (Y1Cre neurons) form an essential pathway in the dorsal spinal cord for the transmission of mechanical but not chemical itch. Ablating or silencing the Y1Cre neurons abrogates mechanical itch, while chemogenetic activation induces scratching. Moreover, using Y1 conditional knockout mice, we demonstrate that endogenous neuropeptide Y (NPY) acts via dorsal-horn Y1-expressing neurons to suppress light punctate touch and mechanical itch stimuli. NPY-Y1 signaling thus regulates the transmission of innocuous tactile information by establishing biologically relevant thresholds for touch discrimination and mechanical itch reflexes.
Keywords: NPY1R signaling; Y1 excitatory neurons; chemical itch; dorsal spinal cord; inhibition; mechanical itch transmission; neuropeptide Y; pain; somatosensory processing; touch.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

