In Vivo Tracking of Tissue Engineered Constructs
- PMID: 31315207
- PMCID: PMC6680880
- DOI: 10.3390/mi10070474
In Vivo Tracking of Tissue Engineered Constructs
Abstract
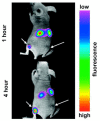
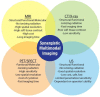
To date, the fields of biomaterials science and tissue engineering have shown great promise in creating bioartificial tissues and organs for use in a variety of regenerative medicine applications. With the emergence of new technologies such as additive biomanufacturing and 3D bioprinting, increasingly complex tissue constructs are being fabricated to fulfill the desired patient-specific requirements. Fundamental to the further advancement of this field is the design and development of imaging modalities that can enable visualization of the bioengineered constructs following implantation, at adequate spatial and temporal resolution and high penetration depths. These in vivo tracking techniques should introduce minimum toxicity, disruption, and destruction to treated tissues, while generating clinically relevant signal-to-noise ratios. This article reviews the imaging techniques that are currently being adopted in both research and clinical studies to track tissue engineering scaffolds in vivo, with special attention to 3D bioprinted tissue constructs.
Keywords: 3D bioprinting; additive manufacturing; bioluminescence; computed tomography (CT); fluorescence spectroscopy; in vivo imaging; magnetic resonant imaging (MRI); magnetic-particle imaging; multimodal imaging; optical coherence tomography; photoacoustic imaging; scaffold tracking; tissue engineering; ultrasound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

