Influenza Virus-Like Particles Presenting both Toxoplasma gondii ROP4 and ROP13 Enhance Protection against T. gondii Infection
- PMID: 31315212
- PMCID: PMC6680409
- DOI: 10.3390/pharmaceutics11070342
Influenza Virus-Like Particles Presenting both Toxoplasma gondii ROP4 and ROP13 Enhance Protection against T. gondii Infection
Abstract
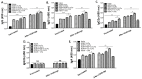
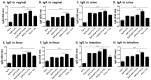
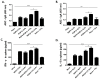
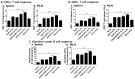
Rhoptry organelle proteins (ROPs) secreted by Toxoplasma gondii (T. gondii) play a critical role during parasite invasion into host cells. In this study, virus-like particles (VLPs) vaccines containing ROP4 and/or ROP13 together with influenza M1 were generated. ROP4+ROP13 VLPs were produced by combining ROP4 VLPs with ROP13 VLPs, and ROP(4 + 13) VLPs by co-infecting insect cells with recombinant baculovirus expressing ROP4 or ROP13. Mice intranasally immunized with ROP(4 + 13) VLPs showed significantly higher levels of IgG, IgG1, IgG2a and IgA antibody responses in sera compared to ROP4+ROP13VLPs. Upon challenge infection by oral route, mice immunized with ROP(4 + 13) VLPs elicited higher levels of IgG and IgA antibody responses in fecal, urine, intestine and vaginal samples as well as CD4+ T, CD8+ T cells, and germinal center B cell responses compared to other type of vaccines, ROP4 VLPs, ROP13 VLPs, and ROP4+ROP13 VLPs. ROP(4 + 13) VLPs vaccination showed a significant decrease in the size and number of cyst in the brain and less body weight loss compared to combination ROP4+ROP13 VLPs upon challenge infection with T. gondii ME49. These results indicated that the ROP(4 + 13) VLPs vaccination provided enhanced protection against T. gondii infection compared to ROP4+ROP13 VLPs, providing an important insight into vaccine design strategy for T. gondii VLPs vaccines.
Keywords: ROP13; ROP4; Toxoplasma gondii; protection; vaccine; virus-like particle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Parasites—Toxoplasmosis (Toxoplasma Infection) Epidemiology & Risk Factors. [(accessed on 4 September 2018)]; Available online: https://www.cdc.gov/parasites/toxoplasmosis/epi.html.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

