In Vitro-In Vivo Correlations Based on In Vitro Dissolution of Parent Drug Diltiazem and Pharmacokinetics of its Metabolite
- PMID: 31315287
- PMCID: PMC6680613
- DOI: 10.3390/pharmaceutics11070344
In Vitro-In Vivo Correlations Based on In Vitro Dissolution of Parent Drug Diltiazem and Pharmacokinetics of its Metabolite
Abstract
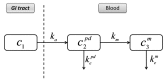
In this study a novel type of in vitro-in vivo correlation (IVIVC) is proposed: The correlation of the in vitro parent drug dissolution data with the in vivo pharmacokinetic data of drug's metabolite after the oral administration of the parent drug. The pharmacokinetic data for the parent drug diltiazem (DTZ) and its desacetyl diltiazem metabolite (DTZM) were obtained from an in vivo study performed in 19 healthy volunteers. The pharmacokinetics of the parent drug and its metabolite followed a pseudomono-compartmental model and deconvolution of the DTZ or DTZM plasma concentration profiles was performed with a Wagner-Nelson-type equation. The calculated in vivo absorption fractions were correlated with the in vitro DTZ dissolution data obtained with USP 2 apparatus. A linear IVIVC was obtained for both DTZ and DTZM, with a better correlation observed for the case of the metabolite. This type of correlation of the in vitro data of the parent compound with the in vivo data of the metabolite could be useful for the development of drugs with active metabolites and prodrugs.
Keywords: diltiazem; dissolution; in vitro–in vivo correlation (IVIVC); mathematical modeling; metabolites.
Conflict of interest statement
Mircioiu, C.; Ionica, G.; Danilceac, A.; Miron, D.; Mircioiu, I.; Radulescu, F.S. Pharmacokinetic and mathematical outliers for drugs with active metabolites. Note i. Model independent analyses for pentoxifylline. FARMACIA 2010, 58, 264–278.
Figures








 ).
).
References
-
- Mahayni H., Rekhi G., Uppoor R., Marroum P., Hussain A., Augsburger L., Eddington N. Evaluation of “External” Predictability of an In Vitro–In Vivo Correlation for an Extended-Release Formulation Containing Metoprolol Tartrate. J. Pharm. Sci. 2000;89:1354–1361. doi: 10.1002/1520-6017(200010)89:10<1354::AID-JPS13>3.0.CO;2-P. - DOI - PubMed
-
- Emami J. In vitro-in vivo correlation: From theory to applications. J. Pharm. Pharm. Sci. 2006;9:169–189. - PubMed
LinkOut - more resources
Full Text Sources

