Extracellular vesicles and soluble factors secreted by Escherichia coli Nissle 1917 and ECOR63 protect against enteropathogenic E. coli-induced intestinal epithelial barrier dysfunction
- PMID: 31315566
- PMCID: PMC6637528
- DOI: 10.1186/s12866-019-1534-3
Extracellular vesicles and soluble factors secreted by Escherichia coli Nissle 1917 and ECOR63 protect against enteropathogenic E. coli-induced intestinal epithelial barrier dysfunction
Abstract
Background: Enteric pathogens have developed mechanisms to disrupt tight junctions and increase gut permeability. Many studies have analysed the ability of live probiotics to protect intestinal epithelial cells against tight junction damage caused by bacterial pathogens. Escherichia coli Nissle 1917 (EcN) is among the probiotics that positively modulates the intestinal epithelial barrier by regulating expression and distribution of tight junction proteins. We previously reported that regulation of ZO-1, claudin-14 and claudin-2 is mediated by EcN secreted factors, either free-released or associated with outer membrane vesicles (OMVs). Factors secreted by commensal ECOR63 elicited comparable effects in intact epithelial T-84 and Caco-2 cell monolayers.
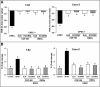
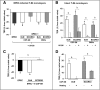
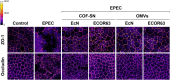
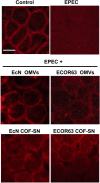
Results: Here we analyse the ability of OMVs and soluble secreted factors to protect epithelial barrier function in polarized T-84 and Caco-2 cells infected with enteropathogenic Escherichia coli (EPEC). Transepithelial electrical resistance, paracellular permeability, mRNA levels and subcellular distribution of tight junction proteins were monitored in the absence or presence of EcN and ECOR63 extracellular fractions. EPEC downregulated expression of ZO-1 ZO-2, occludin and claudin-14 and altered the subcellular localization of ZO-1, occludin and F-actin cytoskeleton. OMVs and soluble factors secreted by EcN and ECOR63 counteracted EPEC-altered transepithelial resistance and paracellular permeability, preserved occludin and claudin-14 mRNA levels, retained ZO-1 and occludin at tight junctions in the cell boundaries and ameliorated F-actin disorganization. Redistribution of ZO-1 was not accompanied by changes at mRNA level.
Conclusion: This study provides new insights on the role of microbiota secreted factors on the modulation of intestinal tight junctions, expanding their barrier-protective effects against pathogen-induced disruption.
Keywords: ECOR63; EPEC; Escherichia coli Nissle 1917; Intestinal epithelial barrier; Outer membrane vesicles; Tight junctions.
Conflict of interest statement
The authors declare that they have no competing interests
Figures