Utility of oral fluid samples for hepatitis B antibody detection in real life conditions
- PMID: 31315573
- PMCID: PMC6637497
- DOI: 10.1186/s12879-019-4183-0
Utility of oral fluid samples for hepatitis B antibody detection in real life conditions
Abstract
Background: Hepatitis B virus (HBV) testing in oral fluid samples may provide advantages in diagnosis, screening or prevalence studies, especially among individuals with venous access difficulties. This study aims to optimize one commercially available assay for detecting total anti-HBc marker in oral fluid samples and to evaluate its utility under real life conditions in different settings for the purposes of prevalence and diagnostic studies.
Methods: Oral fluid was collected using a Salivette device and some parameters were initially evaluated: type of elution buffer and sample volume. Thereafter, the utility of oral fluid samples for detection of anti-HBc was evaluated in real life conditions in which, 1296 individuals gave serum and oral fluid samples. All serum samples were submitted to commercial EIAs to detect total anti-HBc, according to the manufacturer's instructions and oral fluid samples according to previous optimization.
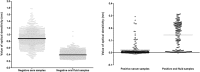
Results: In optimization evaluation, PBS/BSA 0.5% and 100 μL of oral fluid (volume was two-fold increased compared to serum in EIA) were chosen as transport buffer and sample volume. In the field study, anti-HBc was detected in 211 out of 1296 serum samples giving overall oral fluid sensitivity of 52.6% and specificity of 96%. Concordance was higher in ambulatory setting (67.7) compared to general population (31.8). Mean ± standard deviation values of optical density/cutoff (OD/CO) in serum samples were higher in false-negative oral fluid samples than those seen in true positive samples. Sensitivity was higher in those presenting active infection compared to anti-HBc isolate and past infection. Sensitivity also increased in the ambulatory group when HCV individuals were excluded.
Conclusions: It was possible to optimize a commercial EIA for detecting anti-HBc in oral fluid samples and where the highest concordance was found in ambulatory settings and among individuals with active infection.
Keywords: Diagnosis; Enzyme immunoassay; Hepatitis B virus; Oral fluid; Prevalence.
Conflict of interest statement
The authors disclose no actual or potential conflicts of interest, including any financial, personal or other relationships with people or organisations, within 2 years of the beginning of this study that could inappropriately influence the study.
Figures


References
-
- World Health Organization. Hepatitis B. 2017. http://www.who.int/mediacentre/factsheets/fs204/en/ [Accessed on 08.30.17].
-
- Ministry of Health. Boletim Epidemiológico—Hepatites Virais. 2018. Available online: http://www.aids.gov.br/pt-br/pub/2018/boletim-epidemiologico-de-hepatite.... Accessed 10 Sept 2018.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

