Expression of the type 3 InsP3 receptor is a final common event in the development of hepatocellular carcinoma
- PMID: 31315892
- PMCID: PMC7087395
- DOI: 10.1136/gutjnl-2018-317811
Expression of the type 3 InsP3 receptor is a final common event in the development of hepatocellular carcinoma
Erratum in
-
Correction: Expression of the type 3 InsP3 receptor is a final common event in the development of hepatocellular carcinoma.Gut. 2020 Jan;69(1):e1. doi: 10.1136/gutjnl-2018-317811corr1. Gut. 2020. PMID: 31811087 No abstract available.
Abstract
Background & objectives: Hepatocellular carcinoma (HCC) is the second leading cause of cancer death worldwide. Several types of chronic liver disease predispose to HCC, and several different signalling pathways have been implicated in its pathogenesis, but no common molecular event has been identified. Ca2+ signalling regulates the proliferation of both normal hepatocytes and liver cancer cells, so we investigated the role of intracellular Ca2+ release channels in HCC.
Design: Expression analyses of the type 3 isoform of the inositol 1, 4, 5-trisphosphate receptor (ITPR3) in human liver samples, liver cancer cells and mouse liver were combined with an evaluation of DNA methylation profiles of ITPR3 promoter in HCC and characterisation of the effects of ITPR3 expression on cellular proliferation and apoptosis. The effects of de novo ITPR3 expression on hepatocyte calcium signalling and liver growth were evaluated in mice.
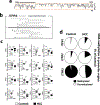
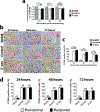
Results: ITPR3 was absent or expressed in low amounts in hepatocytes from normal liver, but was expressed in HCC specimens from three independent patient cohorts, regardless of the underlying cause of chronic liver disease, and its increased expression level was associated with poorer survival. The ITPR3 gene was heavily methylated in control liver specimens but was demethylated at multiple sites in specimens of patient with HCC. Administration of a demethylating agent in a mouse model resulted in ITPR3 expression in discrete areas of the liver, and Ca2+ signalling was enhanced in these regions. In addition, cell proliferation and liver regeneration were enhanced in the mouse model, and deletion of ITPR3 from human HCC cells enhanced apoptosis.
Conclusions: These results provide evidence that de novo expression of ITPR3 typically occurs in HCC and may play a role in its pathogenesis.
Keywords: apoptosis; calcium signaling; cell growth; liver cancer; methylation.
© Author(s) (or their employer(s)) 2019. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

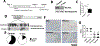

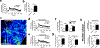
References
-
- Heimbach J, Kulik LM, Finn R, Sirlin CB, Abecassis M, Roberts LR, et al. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology 2017. - PubMed
-
- Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
