Are CAR-T therapies living up to their hype? A study using real-world data in two cohorts to determine how well they are actually working in practice compared with bone marrow transplants
- PMID: 31315904
- PMCID: PMC8165150
- DOI: 10.1136/bmjebm-2019-111226
Are CAR-T therapies living up to their hype? A study using real-world data in two cohorts to determine how well they are actually working in practice compared with bone marrow transplants
Abstract
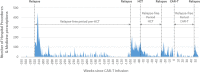
With the increasing use of new regulatory tools, like the Food and Drug Administration's breakthrough designation, there are increasing challenges for European health technology assessors (HTAs) to make an accurate assessment of the long-term value and performance of chimeric antigen receptor T-cell (CAR-T) therapies, particularly for orphan conditions, such as acute lymphoblastic leukaemia. The aim of this study was to demonstrate a novel methodology harnessing longitudinal real-world data, extracted from the electronic health records of a medical centre functioning as a clinical trial site, to develop an accurate analysis of the performance of CAR-T compared with the next-best treatment option, namely allogeneic haematopoietic cell transplant (HCT). The study population comprised 43 subjects in two cohorts: 29 who had undergone HCT treatment and 14 who had undergone CAR-T therapy. The 3-year relapse-free survival probability was 46% (95% CI: 08% to 79%) in the CAR-T cohort and 68% (95% CI: 46% to 83%) in the HCT cohort. To explain the lower RFS probability in the CAR-T cohort compared with the HCT cohort, the authors hypothesised that the CAR-T cohort had a far higher level of disease burden. This was validated by log-rank test analysis (p=0.0001) and confirmed in conversations with practitioners at the study site. The authors are aware that the small populations in this study will be seen as limiting the generalisability of the findings to some readers. However, in consultation with many European HTAs and regulators, there is broad agreement that this methodology warrants further investigation with a larger study.
Keywords: health economics; leukaemia; paediatric oncology; quality in health care.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Walsh F. First child given pioneering CAR-T cancer therapy. BBC News 2019. https://www.bbc.com/news/health-47058069
-
- Novartis Pharmaceuticals Corporation. KYMRIAH (tisagenlecleucel) prescribing information. 2017. Available: https://www.fda.gov/downloads/UCM573941.pdf
-
- Tice J. Chimeric Antigen Receptor T-Cell Therapy for B Cell Cancers: Effectiveness and Value. 2018:6.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
