Yorkie controls tube length and apical barrier integrity during airway development
- PMID: 31315941
- PMCID: PMC6683733
- DOI: 10.1083/jcb.201809121
Yorkie controls tube length and apical barrier integrity during airway development
Erratum in
-
Correction: Yorkie controls tube length and apical barrier integrity during airway development.J Cell Biol. 2023 May 1;222(5):e20180912104072023c. doi: 10.1083/jcb.20180912104072023c. Epub 2023 Apr 13. J Cell Biol. 2023. PMID: 37052885 Free PMC article. No abstract available.
Abstract
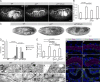
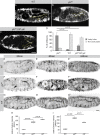
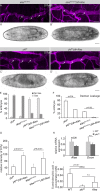
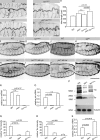
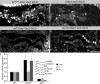
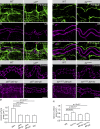
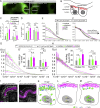
Epithelial organ size and shape depend on cell shape changes, cell-matrix communication, and apical membrane growth. The Drosophila melanogaster embryonic tracheal network is an excellent model to study these processes. Here, we show that the transcriptional coactivator of the Hippo pathway, Yorkie (YAP/TAZ in vertebrates), plays distinct roles in the developing Drosophila airways. Yorkie exerts a cytoplasmic function by binding Drosophila Twinstar, the orthologue of the vertebrate actin-severing protein Cofilin, to regulate F-actin levels and apical cell membrane size, which are required for proper tracheal tube elongation. Second, Yorkie controls water tightness of tracheal tubes by transcriptional regulation of the δ-aminolevulinate synthase gene (Alas). We conclude that Yorkie has a dual role in tracheal development to ensure proper tracheal growth and functionality.
© 2019 Skouloudaki et al.
Figures



References
-
- Beitel G.J., and Krasnow M.A.. 2000. Genetic control of epithelial tube size in the Drosophila tracheal system. Development. 127:3271–3282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

