Structural characterization of an activin class ternary receptor complex reveals a third paradigm for receptor specificity
- PMID: 31315975
- PMCID: PMC6681762
- DOI: 10.1073/pnas.1906253116
Structural characterization of an activin class ternary receptor complex reveals a third paradigm for receptor specificity
Abstract
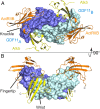
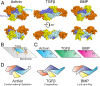
TGFβ family ligands, which include the TGFβs, BMPs, and activins, signal by forming a ternary complex with type I and type II receptors. For TGFβs and BMPs, structures of ternary complexes have revealed differences in receptor assembly. However, structural information for how activins assemble a ternary receptor complex is lacking. We report the structure of an activin class member, GDF11, in complex with the type II receptor ActRIIB and the type I receptor Alk5. The structure reveals that receptor positioning is similar to the BMP class, with no interreceptor contacts; however, the type I receptor interactions are shifted toward the ligand fingertips and away from the dimer interface. Mutational analysis shows that ligand type I specificity is derived from differences in the fingertips of the ligands that interact with an extended loop specific to Alk4 and Alk5. The study also reveals differences for how TGFβ and GDF11 bind to the same type I receptor, Alk5. For GDF11, additional contacts at the fingertip region substitute for the interreceptor interactions that are seen for TGFβ, indicating that Alk5 binding to GDF11 is more dependent on direct contacts. In support, we show that a single residue of Alk5 (Phe84), when mutated, abolishes GDF11 signaling, but has little impact on TGFβ signaling. The structure of GDF11/ActRIIB/Alk5 shows that, across the TGFβ family, different mechanisms regulate type I receptor binding and specificity, providing a molecular explanation for how the activin class accommodates low-affinity type I interactions without the requirement of cooperative receptor interactions.
Keywords: Alk5; GDF11; TGF-β superfamily; activin; ternary signaling complex.
Conflict of interest statement
Conflict of interest statement: T.B.T. is a consultant for Acceleron Pharma and Scientific Founder for Eclode. R.C. and R.K. are current employees of Acceleron Pharma with ownership interest in the company. A.N.E. and V.J.I. are current employees of Regeneron Pharmaceuticals with ownership interest in the company. The other authors report no competing interests.
Figures



References
-
- Hinck A. P., Structural studies of the TGF-betas and their receptors–Insights into evolution of the TGF-beta superfamily. FEBS Lett. 586, 1860–1870 (2012). - PubMed
-
- Weiss A., Attisano L., The TGFbeta superfamily signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2, 47–63 (2013). - PubMed
-
- Attisano L., et al. , Identification of human activin and TGFb type I receptors that form heteromeric kinase complexes with type II receptors. Cell 75, 671–680 (1993). - PubMed
-
- Wrana J. L., Attisano L., Wieser R., Ventura F., Massague J., Mechanism of activation of the TGF-β receptor. Nature 370, 341–347 (1994). - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

