Comparative effectiveness of opioid replacement agents for neonatal opioid withdrawal syndrome: a systematic review and meta-analysis
- PMID: 31316147
- PMCID: PMC7784556
- DOI: 10.1038/s41372-019-0437-3
Comparative effectiveness of opioid replacement agents for neonatal opioid withdrawal syndrome: a systematic review and meta-analysis
Abstract
Objective(s): To compare short-term treatment outcomes of opioid pharmacotherapy for neonatal opioid withdrawal syndrome (NOWS).
Study design: PubMed/MEDLINE, Embase, PsycINFO, and The Cochrane Library were searched from inception through September 30, 2018. Primary outcome was treatment duration (LOT). Secondary outcomes included hospitalization duration (LOS) and rate of adjunct drug needed (RAD).
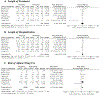
Results: Of 753 publications, 11 studies met inclusion criteria. There was no difference in LOT (WMD -1.39 [-5.79 to -3.01] days, I2 82%) or LOS (WMD -1.48 [-5.75 to -2.79] days, I2 92%) between morphine and methadone. RAD with morphine was higher (RR 1.51 [1.35-1.69], I2 0%). Buprenorphine was associated with shorter LOT (WMD 7.70 [0.88-14.53] days, I2 76%) and LOS (WMD 5.61 [-0.01 to -11.24] days, I2 60%) compared with morphine, in addition to methadone according to two cohort studies.
Conclusions: Methadone had superior primary treatment success compared with morphine. Buprenorphine was associated with the shortest overall durations of treatment and hospitalization.
Conflict of interest statement
Figures


References
-
- Tolia VN, Patrick SW, Bennett MM, Murthy K, Sousa J, Smith PB, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372:2118–26. - PubMed
-
- Patrick SW, Davis MM, Lehman CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatal: Off J Calif Perinat Assoc. 2015;35:667. - PubMed
-
- Kocherlakota P Neonatal abstinence syndrome. Pediatrics. 2014;134:e547–61. - PubMed
-
- Osborn DA, Jeffery HE. Cole, Opiate treatment for opiate withdrawal in newborn infants. Cochrane Datab Syst Rev. 2010;10:Cd002059. - PubMed
-
- Osborn DA, Jeffery HE, Cole MJ. Sedatives for opiate withdrawal in newborn infants. Cochrane Datab Syst Rev. 2010;10: Cd002053. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

