Porcine Invariant Natural Killer T Cells: Functional Profiling and Dynamics in Steady State and Viral Infections
- PMID: 31316500
- PMCID: PMC6611438
- DOI: 10.3389/fimmu.2019.01380
Porcine Invariant Natural Killer T Cells: Functional Profiling and Dynamics in Steady State and Viral Infections
Abstract
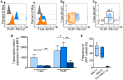
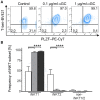
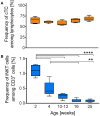
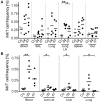
Pigs are important livestock and comprehensive understanding of their immune responses in infections is critical to improve vaccines and therapies. Moreover, similarities between human and swine physiology suggest that pigs are a superior animal model for immunological studies. However, paucity of experimental tools for a systematic analysis of the immune responses in pigs represent a major disadvantage. To evaluate the pig as a biomedical model and additionally expand the knowledge of rare immune cell populations in swine, we established a multicolor flow cytometry analysis platform of surface marker expression and cellular responses for porcine invariant Natural Killer T cells (iNKT). In humans, iNKT cells are among the first line defenders in various tissues, respond to CD1d-restricted antigens and become rapidly activated. Naïve porcine iNKT cells were CD3+/CD4-/CD8+ or CD3+/CD4-/CD8- and displayed an effector- or memory-like phenotype (CD25+/ICOS+/CD5hi/CD45RA-/CCR7 ± /CD27+). Based on their expression of the transcription factors T bet and the iNKT cell-specific promyelocytic leukemia zinc finger protein (PLZF), porcine iNKT cells were differentiated into functional subsets. Analogous to human iNKT cells, in vitro stimulation of porcine leukocytes with the CD1d ligand α-galactosylceramide resulted in rapid iNKT cell proliferation, evidenced by an increase in frequency and Ki-67 expression. Moreover, this approach revealed CD25, CD5, ICOS, and the major histocompatibility complex class II (MHC II) as activation markers on porcine iNKT cells. Activated iNKT cells also expressed interferon-γ, upregulated perforin expression, and displayed degranulation. In steady state, iNKT cell frequency was highest in newborn piglets and decreased with age. Upon infection with two viruses of high relevance to swine and humans, iNKT cells expanded. Animals infected with African swine fever virus displayed an increase of iNKT cell frequency in peripheral blood, regional lymph nodes, and lungs. During Influenza A virus infection, iNKT cell percentage increased in blood, lung lymph nodes, and broncho-alveolar lavage. Our in-depth characterization of porcine iNKT cells contributes to a better understanding of porcine immune responses, thereby facilitating the design of innovative interventions against infectious diseases. Moreover, we provide new evidence that endorses the suitability of the pig as a biomedical model for iNKT cell research.
Keywords: African swine fever virus; T cells; biomedical model; iNKT cells; influenza A virus; pig.
Figures






Similar articles
-
Identification of invariant natural killer T cells in porcine peripheral blood.Vet Immunol Immunopathol. 2012 Oct 15;149(3-4):272-9. doi: 10.1016/j.vetimm.2012.06.023. Epub 2012 Jul 4. Vet Immunol Immunopathol. 2012. PMID: 22939274
-
Characterizing porcine invariant natural killer T cells: A comparative study with NK cells and T cells.Dev Comp Immunol. 2017 Nov;76:343-351. doi: 10.1016/j.dci.2017.07.006. Epub 2017 Jul 8. Dev Comp Immunol. 2017. PMID: 28694168
-
Defining a novel subset of CD1d-dependent type II natural killer T cells using natural killer cell-associated markers.Scand J Immunol. 2019 Sep;90(3):e12794. doi: 10.1111/sji.12794. Epub 2019 Jun 26. Scand J Immunol. 2019. PMID: 31141185 Free PMC article.
-
Harnessing Invariant NKT Cells to Improve Influenza Vaccines: A Pig Perspective.Int J Mol Sci. 2017 Dec 27;19(1):68. doi: 10.3390/ijms19010068. Int J Mol Sci. 2017. PMID: 29280974 Free PMC article. Review.
-
Functions of CD1d-Restricted Invariant Natural Killer T Cells in Antimicrobial Immunity and Potential Applications for Infection Control.Front Immunol. 2018 Jun 6;9:1266. doi: 10.3389/fimmu.2018.01266. eCollection 2018. Front Immunol. 2018. PMID: 29928278 Free PMC article. Review.
Cited by
-
Time Series Transcriptomic Analysis of Bronchoalveolar Lavage Cells from Piglets Infected with Virulent or Low-Virulent Porcine Reproductive and Respiratory Syndrome Virus 1.J Virol. 2022 Feb 9;96(3):e0114021. doi: 10.1128/JVI.01140-21. Epub 2021 Dec 1. J Virol. 2022. PMID: 34851149 Free PMC article.
-
Investigation of activation-induced markers (AIM) in porcine T cells by flow cytometry.Front Vet Sci. 2024 May 29;11:1390486. doi: 10.3389/fvets.2024.1390486. eCollection 2024. Front Vet Sci. 2024. PMID: 38868498 Free PMC article.
-
Unaltered influenza disease outcomes in swine prophylactically treated with α-galactosylceramide.Dev Comp Immunol. 2021 Jan;114:103843. doi: 10.1016/j.dci.2020.103843. Epub 2020 Aug 29. Dev Comp Immunol. 2021. PMID: 32871161 Free PMC article.
-
Bovine Colostrum Before or After Formula Feeding Improves Systemic Immune Protection and Gut Function in Newborn Preterm Pigs.Front Immunol. 2020 Jan 30;10:3062. doi: 10.3389/fimmu.2019.03062. eCollection 2019. Front Immunol. 2020. PMID: 32082298 Free PMC article.
-
Crosstalk between trace elements and T-cell immunity during early-life health in pigs.Sci China Life Sci. 2023 Sep;66(9):1994-2005. doi: 10.1007/s11427-022-2339-0. Epub 2023 Jun 6. Sci China Life Sci. 2023. PMID: 37300752 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

