From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived From the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit
- PMID: 31316537
- PMCID: PMC6609884
- DOI: 10.3389/fpls.2019.00835
From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived From the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit
Abstract
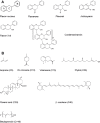
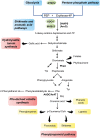
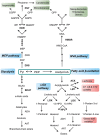
Fruit flavor and nutritional characteristics are key quality traits and ones of the main factors influencing consumer preference. Central carbon metabolism, also known as primary metabolism, contributes to the synthesis of intermediate compounds that act as precursors for plant secondary metabolism. Specific and specialized metabolic pathways that evolved from primary metabolism play a key role in the plant's interaction with its environment. In particular, secondary metabolites present in the fruit serve to increase its attractiveness to seed dispersers and to protect it against biotic and abiotic stresses. As a consequence, several important organoleptic characteristics, such as aroma, color, and fruit nutritional value, rely upon secondary metabolite content. Phenolic and terpenoid compounds are large and diverse classes of secondary metabolites that contribute to fruit quality and have their origin in primary metabolic pathways, while the delicate aroma of ripe fruits is formed by a unique combination of hundreds of volatiles that are derived from primary metabolites. In this review, we show that the manipulation of primary metabolism is a powerful tool to engineer quality traits in fruits, such as the phenolic, terpenoid, and volatile content. The enzymatic reactions responsible for the accumulation of primary precursors are bottlenecks in the transfer of metabolic flux from central to specialized metabolism and should be taken into account to increase the yield of the final products of the biosynthetic pathways. In addition, understanding the connection and regulation of the carbon flow between primary and secondary metabolism is a key factor for the development of fruit cultivars with enhanced organoleptic and nutritional traits.
Keywords: flavor; fruit; metabolic engineering; primary metabolism; quality traits; secondary metabolism.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

