Impact of first-line infliximab on the pharmacokinetics of second-line vedolizumab in inflammatory bowel diseases
- PMID: 31316779
- PMCID: PMC6620879
- DOI: 10.1177/2050640619841538
Impact of first-line infliximab on the pharmacokinetics of second-line vedolizumab in inflammatory bowel diseases
Erratum in
-
Erratum.United European Gastroenterol J. 2019 Aug;7(7):988. doi: 10.1177/2050640619865821. Epub 2019 Jul 31. United European Gastroenterol J. 2019. PMID: 31428426 Free PMC article.
Abstract
Background: Very little is known about the impact of the wash-out period on the pharmacokinetics of a second-line biologic.
Objective: The objective of this article is to explore the impact of two different wash-out periods on the pharmacokinetics of vedolizumab and infliximab.
Methods: Patients switching from infliximab to vedolizumab were retrospectively identified. The population was divided into two groups according to wash-out period: <6 weeks or >6 weeks. Vedolizumab and infliximab trough levels (TLs) were determined and correlated with clinical and biological outcomes.
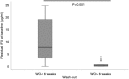
Results: A total of 71 inflammatory bowel disease patients were included. At week 6, in patients previously treated with infliximab, median vedolizumab TLs were 21.9 µg/ml and 24.9 µg/ml for the <6 weeks and >6 weeks wash-out period, respectively (p = 0.31), whereas median residual infliximab TLs were 0.5 µg/ml and 0 µg/ml (p = 0.034). The rate of treatment discontinuation was similar (p = 0.64), and the infectious events were six and two for the <6 weeks and >6 weeks wash-out period, respectively (p = 0.12) by week 30.
Conclusions: This study suggests clinicians may not need to be concerned about the impact of wash-out period on the pharmacokinetics of the second-line biologic when switching infliximab to vedolizumab. More data are required on the impact of wash-out period on safety.
Keywords: Induction; inflammatory bowel disease; infliximab; pharmacokinetics; trough level; vedolizumab; wash-out.
Figures



References
-
- Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today 2006; 11: 81–88. - PubMed
-
- Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2008; 84: 548–558. - PubMed
-
- Cohen-Solal JF, Cassard L, Fridman WH, et al. Fc gamma receptors. Immunol Lett 2004; 92: 199–205. - PubMed
-
- Suzuki T, Ishii-Watabe A, Tada M, et al. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: A comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunol 2010; 184: 1968–1976. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

