Etelcalcetide Is Effective at All Levels of Severity of Secondary Hyperparathyroidism in Hemodialysis Patients
- PMID: 31317120
- PMCID: PMC6611952
- DOI: 10.1016/j.ekir.2019.04.010
Etelcalcetide Is Effective at All Levels of Severity of Secondary Hyperparathyroidism in Hemodialysis Patients
Abstract
Introduction: Calcimimetics improve parameters of secondary hyperparathyroidism (sHPT) but are mostly initiated when patients have severe disease, potentially limiting effectiveness. We evaluated the effects of etelcalcetide on lowering intact parathyroid hormone, calcium, and phosphate at different disease severity levels.
Methods: This analysis examined data from 2 parallel, phase 3, randomized, placebo-controlled, 26-week trials conducted in 1023 adult (≥18 years old) patients with sHPT on maintenance hemodialysis. Etelcalcetide effects by baseline intact parathyroid hormone stratum (<600, 600-1000, and >1000 ng/l) on mean percentage change in intact parathyroid hormone; changes in calcium and phosphate; and achieving serum intact parathyroid hormone ≤300 ng/l, phosphate <1.78 mmol/l, and both combined, were assessed.
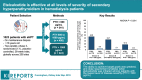
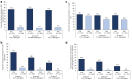
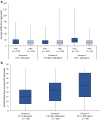
Results: Etelcalcetide reduced serum intact parathyroid hormone by a similar percentage across baseline strata. A similar proportion achieved >30% intact parathyroid hormone reduction across strata for the etelcalcetide arms. Parathyroid hormone increased modestly in each placebo-group stratum, most prominently in the lowest stratum. Serum calcium and phosphate concentrations decreased across strata in etelcalcetide-treated patients, with the most pronounced reductions in patients with highest baseline parathyroid hormone. However, the proportion of patients achieving parathyroid hormone, phosphate, and both targets was highest in the lowest baseline parathyroid hormone stratum, where etelcalcetide dose requirements were lowest. Etelcalcetide dose requirement was lowest among patients in the lowest intact parathyroid hormone stratum.
Conclusion: Etelcalcetide effectively lowered serum intact parathyroid hormone, calcium, and phosphate, irrespective of the severity of secondary hyperparathyroidism. The ability to achieve target goals was greatest, and dose requirement smallest, when etelcalcetide was initiated among patients with the lowest level of disease severity.
Keywords: calcium; chronic kidney disease; hemodialysis; mineral metabolism; parathyroid hormone; phosphate.
Figures
References
-
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2017;7:1–59. - PMC - PubMed
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1-59. - PMC - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO Clinical Practice Guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009;113:S1–S130. - PubMed
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO Clinical Practice Guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1-S130. - PubMed
-
- Bover J., Ureña P., Ruiz-Garcia C. Clinical and practical use of calcimimetics in dialysis patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2016;11:161–174. - PMC - PubMed
- Bover J, Ureña P, Ruiz-Garcia C, et al. Clinical and practical use of calcimimetics in dialysis patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2016;11:161-174. - PMC - PubMed
-
- Frãzao J., Rodriguez M. Secondary hyperparathyroidism disease stabilization following calcimimetic therapy. NDT Plus. 2008;1(suppl 1):i12–i17. - PMC - PubMed
- Frazao J, Rodriguez M. Secondary hyperparathyroidism disease stabilization following calcimimetic therapy. NDT Plus. 2008;1(suppl 1):i12-i17. - PMC - PubMed
-
- Block G.A., Bushinsky D.A., Cunningham J. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA. 2017;317:146–155. - PubMed
- Block GA, Bushinsky DA, Cunningham J, et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA. 2017;317:146-155. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

