Current perspectives on biosimilars
- PMID: 31317293
- PMCID: PMC6791907
- DOI: 10.1007/s10295-019-02216-z
Current perspectives on biosimilars
Abstract
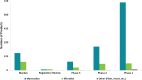
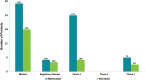
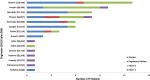
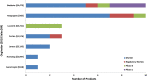
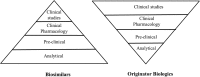
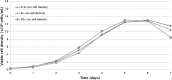
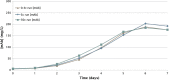
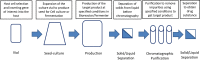
In this work, an overview of the biosimilars market, pipeline and industry targets is discussed. Biosimilars typically have a shorter timeline for approval (8 years) compared to 12 years for innovator drugs and the development cost can be 10-20% of the innovator drug. The biosimilar pipeline is reviewed as well as the quality management system (QMS) that is needed to generate traceable, trackable data sets. One difference between developing a biosimilar compared to an originator is that a broader analytical foundation is required for biosimilars and advances made in developing analytical similarity to characterize these products are discussed. An example is presented on the decisions and considerations explored in the development of a biosimilar and includes identification of the best process parameters and methods based on cost, time, and titer. Finally factors to consider in the manufacture of a biosimilar and approaches used to achieve the target-directed development of a biosimilar are discussed.
Keywords: Analytical; Biosimilars; Mammalian; Manufacturing; Microbial; Quality.
Figures

References
-
- ACE (2018) ACE Software. http://www.pscsoftware.com/software/ACE/overview. Accessed 30 Oct 2018
-
- Alten R, Cronstein BN. Clinical trial development for biosimilars. Semin Arthritis Rheum. 2015;44(2015):S2–S8. - PubMed
-
- Biosimilars Council: A Division of AAM (2018) New Study Finds Biosimilar Competition Can Lower Prescription Drug Costs [Internet]. Washington DC: Association for Accessible Medicines 2018 July 10 [cited 2019 May 7]. [About 3 screens]. https://www.biosimilarscouncil.org/resource/new-study-finds-biosimilar-c.... Accessed 7 May 2019
-
- Biosimilar medicines: Overview [Internet]. London (UK): European Medicines Agency; c1995–2018 [cited 2019 Jan 5]. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/general/gene.... Accessed 5 Jan 2019
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

