Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction
- PMID: 31318148
- PMCID: PMC6718575
- DOI: 10.1111/acel.13004
Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction
Erratum in
-
Corrigendum to: Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction.Aging Cell. 2023 May;22(5):e13816. doi: 10.1111/acel.13816. Epub 2023 Apr 8. Aging Cell. 2023. PMID: 37029569 Free PMC article. No abstract available.
Abstract
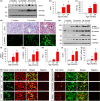
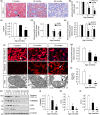
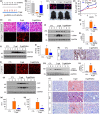
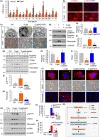
Renal fibrosis is the common pathological feature in a variety of chronic kidney diseases. Aging is highly associated with the progression of renal fibrosis. Among several determinants, mitochondrial dysfunction plays an important role in aging. However, the underlying mechanisms of mitochondrial dysfunction in age-related renal fibrosis are not elucidated. Herein, we found that Wnt/β-catenin signaling and renin-angiotensin system (RAS) activity were upregulated in aging kidneys. Concomitantly, mitochondrial mass and functions were impaired with aging. Ectopic expression of Klotho, an antagonist of endogenous Wnt/β-catenin activity, abolished renal fibrosis in d-galactose (d-gal)-induced accelerated aging mouse model and significantly protected renal mitochondrial functions by preserving mass and diminishing the production of reactive oxygen species. In an established aging mouse model, dickkopf 1, a more specific Wnt inhibitor, and the mitochondria-targeted antioxidant mitoquinone restored mitochondrial mass and attenuated tubular senescence and renal fibrosis. In a human proximal tubular cell line (HKC-8), ectopic expression of Wnt1 decreased biogenesis and induced dysfunction of mitochondria, and triggered cellular senescence. Moreover, d-gal triggered the transduction of Wnt/β-catenin signaling, which further activated angiotensin type 1 receptor (AT1), and then decreased the mitochondrial mass and increased cellular senescence in HKC-8 cells and primary cultured renal tubular cells. These effects were inhibited by AT1 blocker of losartan. These results suggest inhibition of Wnt/β-catenin signaling and the RAS could slow the onset of age-related mitochondrial dysfunction and renal fibrosis. Taken together, our results indicate that Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction.
Keywords: Wnt/β-catenin; aging; mitochondrial dysfunction; renal fibrosis.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures




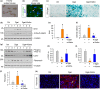
References
-
- Bobkova, N. V. , Evgen'ev, M. , Garbuz, D. G. , Kulikov, A. M. , Morozov, A. , Samokhin, A. , … Nudler, E. (2015). Exogenous Hsp70 delays senescence and improves cognitive function in aging mice. Proceedings of the National Academy of Sciences USA, 112, 16006–16011. 10.1073/pnas.1516131112 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81722011/National Natural Science Foundation of China/International
- 81521003/National Natural Science Foundation of China/International
- 2018GZR110105004/Frontier Research Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory/International
- 2018GZR110102004/Outstanding Scholar Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory/International
LinkOut - more resources
Full Text Sources
Medical
Research Materials

