Formulation technologies for oral vaccines
- PMID: 31318446
- PMCID: PMC6797897
- DOI: 10.1111/cei.13352
Formulation technologies for oral vaccines
Abstract
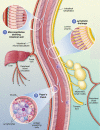
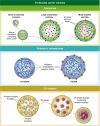
Many options now exist for constructing oral vaccines which, in experimental systems, have shown themselves to be able to generate highly effective immunity against infectious diseases. Their suitability for implementation in clinical practice, however, for prevention of outbreaks, particularly in low- and middle-income countries (LMIC), is not always guaranteed, because of factors such as cost, logistics and cultural and environmental conditions. This brief overview provides a summary of the various approaches which can be adopted, and evaluates them from a pharmaceutical point, taking into account potential regulatory issues, expense, manufacturing complexity, etc., all of which can determine whether a vaccine approach will be successful in the late stages of development. Attention is also drawn to problems arising from inadequate diet, which impacts upon success in stimulating effective immunity, and identifies the use of lipid-based carriers as a way to counteract the problem of nutritional deficiencies in vaccination campaigns.
Keywords: Peyer’s patch; delivery; oral; vaccine.
© 2019 The Authors. Clinical & Experimental Immunology published by John Wiley & Sons Ltd on behalf of British Society for Immunology.
Figures
References
-
- Pandit J, Sultana Y, Kalam MA. Newer technologies in oral vaccine delivery. Current Drug Therapy 2014; 9:173–87.
-
- Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol 2009; 183:6883–92. - PubMed
-
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 2003; 3:331–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

