Hydrogen Bond Enhanced Halogen Bonds: A Synergistic Interaction in Chemistry and Biochemistry
- PMID: 31318520
- PMCID: PMC7328900
- DOI: 10.1021/acs.accounts.9b00189
Hydrogen Bond Enhanced Halogen Bonds: A Synergistic Interaction in Chemistry and Biochemistry
Abstract
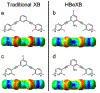
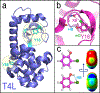
The halogen bond (XB) has become an important tool for molecular design in all areas of chemistry, including crystal and materials engineering and medicinal chemistry. Its similarity to the hydrogen bond (HB) makes the relationship between these interactions complex, at times competing against and other times orthogonal to each other. Recently, our two laboratories have independently reported and characterized a synergistic relationship, in which the XB is enhanced through direct intramolecular HBing to the electron-rich belt of the halogen. In one study, intramolecular HBing from an amine polarizes the iodopyridinium XB donors of a bidentate anion receptor. The resulting HB enhanced XB (or HBeXB) preorganizes and further augments the XB donors. Consequently, the affinity of the receptor for halogen anions was significantly increased. In a parallel study, a meta-chlorotyrosine was engineered into T4 lysozyme, resulting in a HBeXB that increased the thermal stability and activity of the enzyme at elevated temperatures. The crystal structure showed that the chlorine of the noncanonical amino acid formed a XB to the protein backbone, which augmented the HB of the wild-type enzyme. Calorimetric analysis resulted in an enthalpic contribution of this Cl-XB to the stability of the protein that was an order of magnitude greater than previously determined in biomolecules. Quantum mechanical (QM) calculations showed that rotating the hydroxyl group of the tyrosine to point toward rather than away from the halogen greatly increased its potential to serve as a XB donor, equivalent to what was observed experimentally. In sum, the two systems described here show that the HBeXB concept extends the range of interaction energies and geometries to be significantly greater than that of the XB alone. Additionally, surveys of structural databases indicate that the components for this interaction are already present in many existing molecular systems. The confluence of the independent studies from our two laboratories demonstrates the reach of the HBeXB across both chemistry and biochemistry and that intentional engineering of this enhanced interaction will extend the applications of XBs beyond these two initial examples.
Figures
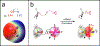
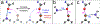





References
-
- Latimer WM; Rodebush WH Polarity and Ionization from the Standpoint of the Lewis Theory of Valence. J. Am. Chem. Soc. 1920, 42, 1419–1433.
-
- Pauling LCN-QP 1960. The Nature of the Chemical Bond and the Structure of Molecules and Crystals; an Introduction to Modern Structural Chemistry, 3d ed.; Cornell University Press: Ithaca, N.Y., 1960.
-
- Smith DA A Brief History of the Hydrogen Bond In Modeling th Hydrogen Bond (ACS Symposium Series); Smith DA, Ed.; American Chemical Society: Washington DC, 1994; pp 1–4.
-
- Pimentel GC; McClellan AL The Hydrogen Bond; W. H. Freeman and Co.: San Francisco, 1960.
-
- Baker EN Hydrogen Bonding in Biological Macromolecules In International Tables for Crystallography Volume F: Crystallography of biological macromolecules; Rossmann MG, Arnold E, Eds.; Springer; Netherlands: Dordrecht, 2001; pp 546–552.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

