AhR-ROR-γt complex is a therapeutic target for MAP4K3/GLKhighIL-17Ahigh subpopulation of systemic lupus erythematosus
- PMID: 31318609
- PMCID: PMC6766655
- DOI: 10.1096/fj.201900105RR
AhR-ROR-γt complex is a therapeutic target for MAP4K3/GLKhighIL-17Ahigh subpopulation of systemic lupus erythematosus
Abstract
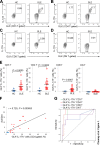
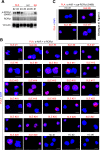
The cytokine IL-17A plays critical roles in the pathogenesis of autoimmune diseases. The frequencies of MAP kinase kinase kinase kinase 3 [also named germinal center kinase-like kinase (GLK)]-overexpressing T cells are correlated with disease severity of systemic lupus erythematosus (SLE). T-cell-specific GLK-transgenic mice develop spontaneous autoimmune responses through IL-17A. GLK signaling selectively stimulates IL-17A production in murine T cells through inducing aryl hydrocarbon receptor (AhR)-retinoic acid receptor-related orphan nuclear receptor-γt (ROR-γt) complex formation. Here, we investigated whether GLK-induced AhR-ROR-γt complex in T cells is a therapeutic target for human SLE. The population of GLK+IL-17A+ T cells was enhanced in the peripheral blood from patients with SLE compared with that of healthy controls using flow cytometry. The receiver operating characteristic curve analysis showed that increased GLK+IL-17A+ T-cell population in peripheral blood reflected an active stage of SLE. In addition, peripheral blood T cells from patients with SLE displayed induction of ROR-γt phosphorylation and the AhR-ROR-γt (and AhR-phosphorylated ROR-γt) complex. Moreover, we identified a small-molecule inhibitor, verteporfin, that inhibited GLK kinase activity and AhR-ROR-γt interaction. The small-molecule inhibitor verteporfin suppressed the disease severity in autoimmune mouse models and IL-17A production in T cells from patients with SLE. Collectively, the GLK-induced AhR-ROR-γt (and AhR-phosphorylated ROR-γt) complex is a therapeutic target for the GLKhighIL-17Ahigh subpopulation of human patients with SLE.-Chuang, H.-C., Chen, Y.-M., Chen, M.-H., Hung, W.-T., Yang, H.-Y., Tseng, Y.-H., Tan, T.-H. AhR-ROR-γt complex is a therapeutic target for MAP4K3/GLKhighIL-17Ahigh subpopulation of systemic lupus erythematosus.
Keywords: SLE; autoimmune disease; verteporfin.
Conflict of interest statement
The authors thank the Laboratory Animal Center (Association for Assessment and Accreditation of Laboratory Animal Care
Figures




References
-
- Martin J. C., Baeten D. L., Josien R. (2014) Emerging role of IL-17 and Th17 cells in systemic lupus erythematosus. Clin. Immunol. 154, 1–12 - PubMed
-
- Yang J., Chu Y., Yang X., Gao D., Zhu L., Yang X., Wan L., Li M. (2009) Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 60, 1472–1483 - PubMed
-
- Henriques A., Inês L., Couto M., Pedreiro S., Santos C., Magalhães M., Santos P., Velada I., Almeida A., Carvalheiro T., Laranjeira P., Morgado J. M., Pais M. L., da Silva J. A., Paiva A. (2010) Frequency and functional activity of Th17, Tc17 and other T-cell subsets in systemic lupus erythematosus. Cell. Immunol. 264, 97–103 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

