A Dried Blood Spot Analysis for Solithromycin in Adolescents, Children, and Infants: A Short Communication
- PMID: 31318840
- PMCID: PMC6856424
- DOI: 10.1097/FTD.0000000000000670
A Dried Blood Spot Analysis for Solithromycin in Adolescents, Children, and Infants: A Short Communication
Abstract
Background: Solithromycin is a fourth-generation macrolide antibiotic with potential efficacy in pediatric community-acquired bacterial pneumonia. Pharmacokinetic (PK) studies of solithromycin in pediatric subjects are limited, therefore application of minimally invasive drug sampling techniques, such as dried blood spots (DBS), may enhance the enrollment of children in PK studies. The objectives of this study were to compare solithromycin concentrations in DBS with those in liquid plasma samples (LPS) and to quantify the effects of modeling DBS concentrations on the results of a population PK model.
Methods: Comparability analysis was performed on matched DBS and LPS solithromycin concentrations collected from two different phase 1 clinical trials of solithromycin treatment in children (clinicaltrials.gov #NCT01966055 and #NCT02268279). Comparability of solithromycin concentrations was evaluated based on DBS:LPS ratio, median percentage prediction error, and median absolute percentage prediction error. The effect of correcting DBS concentrations for both hematocrit and protein binding was investigated. In addition, a previously published population PK model (NONMEM) was leveraged to compare parameter estimates resulting from either DBS or LPS concentrations.
Results: A total of 672 paired DBS-LPS concentrations were available from 95 subjects (age: 0-17 years of age). The median (range) LPS and DBS solithromycin concentrations were 0.3 (0.01-12) mcg/mL and 0.32 (0.01-14) mcg/mL, respectively. Median percentage prediction error and median absolute percentage prediction error of raw DBS to LPS solithromycin concentrations were 5.26% and 22.95%, respectively. In addition, the majority of population PK parameter estimates resulting from modeling DBS concentrations were within 15% of those obtained from modeling LPS concentrations.
Conclusions: Solithromycin concentrations in DBS were similar to those measured in LPS and did not require correction for hematocrit or protein binding.
Figures
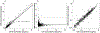
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN267200700051C/HD/NICHD NIH HHS/United States
- K23 HD083465/HD/NICHD NIH HHS/United States
- K24 AI143971/AI/NIAID NIH HHS/United States
- HHSN275201000003C/HD/NICHD NIH HHS/United States
- HHSN275201000003I/HD/NICHD NIH HHS/United States
- T32 GM086330/GM/NIGMS NIH HHS/United States
- K23 HD090239/HD/NICHD NIH HHS/United States
- UL1 TR001117/TR/NCATS NIH HHS/United States
- R01 HD076676/HD/NICHD NIH HHS/United States
- HHSN272201300017C/AI/NIAID NIH HHS/United States
- HHSN272201300017I/AI/NIAID NIH HHS/United States
- HHSN272201500006C/AI/NIAID NIH HHS/United States
- U18 FD006298/FD/FDA HHS/United States

