B lymphocytes directly contribute to tissue fibrosis in patients with IgG4-related disease
- PMID: 31319101
- PMCID: PMC6960365
- DOI: 10.1016/j.jaci.2019.07.004
B lymphocytes directly contribute to tissue fibrosis in patients with IgG4-related disease
Abstract
Background: IgG4-related disease (IgG4-RD) is a fibroinflammatory condition marked by rapid clinical improvement after selective depletion of B lymphocytes with rituximab. This feature suggests that B cells might participate in fibrogenesis and wound healing.
Objective: In the present work we aimed to demonstrate that B lymphocytes contribute directly to tissue fibrosis in patients with IgG4-RD.
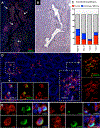
Methods: Total circulating CD19+ B lymphocytes, naive B cells, memory B cells, or plasmablasts from patients with IgG4-RD were cultivated with human fibroblasts. Profibrotic soluble factors and collagen production in cocultures were assessed by using ELISAs and Luminex assays. RNA sequencing and quantitative RT-PCR were used to assess fibroblast activation in the presence of B cells, as well as induction of profibrotic pathways in B-cell subsets. Relevant profibrotic and inflammatory molecules were confirmed in vitro by using functional experiments and on IgG4-RD tissue sections by using multicolor immunofluorescence studies.
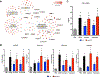
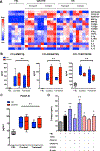
Results: B cells from patients with IgG4-RD (1) produced the profibrotic molecule platelet-derived growth factor B and stimulated collagen production by fibroblasts; (2) expressed enzymes implicated in extracellular matrix remodeling, such as lysyl oxidase homolog 2; (3) produced the chemotactic factors CCL4, CCL5, and CCL11; and (4) induced production of these same chemokines by activated fibroblasts. Plasmablasts expressed sets of genes implicated in fibroblast activation and proliferation and therefore represent cells with intrinsic profibrotic properties.
Conclusion: We have demonstrated that B cells contribute directly to tissue fibrosis in patients with IgG4-RD. These unanticipated profibrotic properties of B lymphocytes, particularly plasmablasts, might be relevant for fibrogenesis in patients with other fibroinflammatory disorders and for wound-healing processes in physiologic conditions.
Keywords: B cells; IgG(4); IgG(4)-related disease; fibroblasts; fibrosis; lysyl oxidase homolog 2; plasmablasts; platelet-derived growth factor; rituximab.
Copyright © 2019 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



Comment in
-
New paradigm of B-cell biology regarding the elucidation of a new mechanism of tissue fibrosis in IgG4-related disease.J Allergy Clin Immunol. 2020 Mar;145(3):785-787. doi: 10.1016/j.jaci.2020.01.004. Epub 2020 Jan 16. J Allergy Clin Immunol. 2020. PMID: 31954774 No abstract available.
References
-
- Rockey DC, Bell PD, Hill JA. Fibrosis-a Common Pathway to Organ Injury and Failure. NEJM. 2015;372:1138–49. - PubMed
-
- Ho YY, Lagares D, Tager AM, Kapoor M. Fibrosis-a lethal component of systemic sclerosis. Nat Rev Rheumatol. 2014;10:390–402. - PubMed
-
- Carruthers MN, Topazian MD, Khosroshahi A, Witzig TE, Wallace ZS, Hart PA, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015;74:1171–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

