Effect of sleep deprivation and exercise on reaction threshold in adults with peanut allergy: A randomized controlled study
- PMID: 31319102
- PMCID: PMC6904229
- DOI: 10.1016/j.jaci.2019.06.038
Effect of sleep deprivation and exercise on reaction threshold in adults with peanut allergy: A randomized controlled study
Abstract
Background: Peanut allergy causes severe and fatal reactions. Current food allergen labeling does not address these risks adequately against the burden of restricting food choice for allergic patients because of limited data on thresholds of reactivity and the influence of everyday factors.
Objective: We estimated peanut threshold doses for a United Kingdom population with peanut allergy and examined the effect of sleep deprivation and exercise.
Methods: In a crossover study, after blind challenge, participants with peanut allergy underwent 3 open peanut challenges in random order: with exercise after each dose, with sleep deprivation preceding challenge, and with no intervention. Primary outcome was the threshold dose triggering symptoms (in milligrams of protein). Primary analysis estimated the difference between the nonintervention challenge and each intervention in log threshold (as percentage change). Dose distributions were modeled, deriving eliciting doses in the population with peanut allergy.
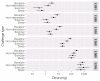
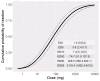
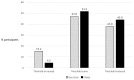
Results: Baseline challenges were performed in 126 participants, 100 were randomized, and 81 (mean age, 25 years) completed at least 1 further challenge. The mean threshold was 214 mg (SD, 330 mg) for nonintervention challenges, and this was reduced by 45% (95% CI, 21% to 61%; P = .001) and 45% (95% CI, 22% to 62%; P = .001) for exercise and sleep deprivation, respectively. Mean estimated eliciting doses for 1% of the population were 1.5 mg (95% CI, 0.8-2.5 mg) during nonintervention challenge (n = 81), 0.5 mg (95% CI, 0.2-0.8 mg) after sleep, and 0.3 mg (95% CI, 0.1-0.6 mg) after exercise.
Conclusion: Exercise and sleep deprivation each significantly reduce the threshold of reactivity in patients with peanut allergy, putting them at greater risk of a reaction. Adjusting reference doses using these data will improve allergen risk management and labeling to optimize protection of consumers with peanut allergy.
Trial registration: ClinicalTrials.gov NCT01429896.
Keywords: Peanut; allergy; exercise and sleep deprivation; thresholds.
Copyright © 2019. Published by Elsevier Inc.
Conflict of interest statement
Funded by the Food Standards Agency who had no involvement in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. None of the authors had a conflict of interest relevant to this study.
Figures



Similar articles
-
Effects of Exercise and Sleep Deprivation on Reaction Severity During Oral Peanut Challenge: A Randomized Controlled Trial.J Allergy Clin Immunol Pract. 2022 Sep;10(9):2404-2413.e1. doi: 10.1016/j.jaip.2022.04.043. Epub 2022 May 25. J Allergy Clin Immunol Pract. 2022. PMID: 35623576 Clinical Trial.
-
Peanut Allergen Threshold Study (PATS): Novel single-dose oral food challenge study to validate eliciting doses in children with peanut allergy.J Allergy Clin Immunol. 2017 May;139(5):1583-1590. doi: 10.1016/j.jaci.2017.01.030. Epub 2017 Feb 24. J Allergy Clin Immunol. 2017. PMID: 28238744
-
The distribution of individual threshold doses eliciting allergic reactions in a population with peanut allergy.J Allergy Clin Immunol. 2002 Dec;110(6):915-20. doi: 10.1067/mai.2002.129235. J Allergy Clin Immunol. 2002. PMID: 12464959 Clinical Trial.
-
Using data from food challenges to inform management of consumers with food allergy: A systematic review with individual participant data meta-analysis.J Allergy Clin Immunol. 2021 Jun;147(6):2249-2262.e7. doi: 10.1016/j.jaci.2021.01.025. Epub 2021 Feb 9. J Allergy Clin Immunol. 2021. PMID: 33571537 Free PMC article.
-
Study of induction of Tolerance to Oral Peanut: a randomised controlled trial of desensitisation using peanut oral immunotherapy in children (STOP II).Southampton (UK): NIHR Journals Library; 2014 Dec. Southampton (UK): NIHR Journals Library; 2014 Dec. PMID: 25642539 Free Books & Documents. Review.
Cited by
-
Precision medicine reaching out to the patients in allergology - a German-Japanese workshop report.Allergol Select. 2021 May 27;5:162-179. doi: 10.5414/ALX02234E. eCollection 2021. Allergol Select. 2021. PMID: 34079922 Free PMC article.
-
Weighing the benefits and risks of oral immunotherapy in clinical practice.Allergy Asthma Proc. 2021 Mar 1;42(2):118-123. doi: 10.2500/aap.2021.42.200107. Allergy Asthma Proc. 2021. PMID: 33685555 Free PMC article.
-
Risk factors for severe reactions in food allergy: Rapid evidence review with meta-analysis.Allergy. 2022 Sep;77(9):2634-2652. doi: 10.1111/all.15318. Epub 2022 Apr 28. Allergy. 2022. PMID: 35441718 Free PMC article. Review.
-
Update of the S2k guideline on the management of IgE-mediated food allergies.Allergol Select. 2021 Jul 8;5:195-243. doi: 10.5414/ALX02257E. eCollection 2021. Allergol Select. 2021. PMID: 34263109 Free PMC article.
-
Reaction phenotypes in IgE-mediated food allergy and anaphylaxis.Ann Allergy Asthma Immunol. 2020 May;124(5):473-478. doi: 10.1016/j.anai.2019.12.023. Epub 2020 Jan 7. Ann Allergy Asthma Immunol. 2020. PMID: 31923546 Free PMC article. Review.
References
-
- Pumphrey RSH. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30(8):1144–50. - PubMed
-
- Pumphrey RS, Gowland MH. Further fatal allergic reactions to food in the United Kingdom 1999-2006. J Allergy Clin Immunol. 2007;119:1018–9. - PubMed
-
- Stiefel G, Anagnostou K, Boyle RJ, Brathwaite N, Ewan P, Fox AT, et al. BSACI guideline for the diagnosis and management of peanut and tree nut allergy. Clin Exp Allergy. 2017;47(6) - PubMed
-
- Remington BC, Baumert JL, Blom WM, Houben GF, Taylor SL, Kruizinga AG. Unintended allergens in precautionary labelled and unlabelled products pose significant risks to UK allergic consumers. Allergy Eur J Allergy Clin Immunol. 2015 - PubMed

